Compositions and methods for cardiac regeneration
A heart tissue and polymer technology, applied in the direction of drug combination, non-active ingredients of polymer compounds, drug delivery, etc., can solve the problems of limited ability of myocardial cell renewal, damage of heart tissue, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
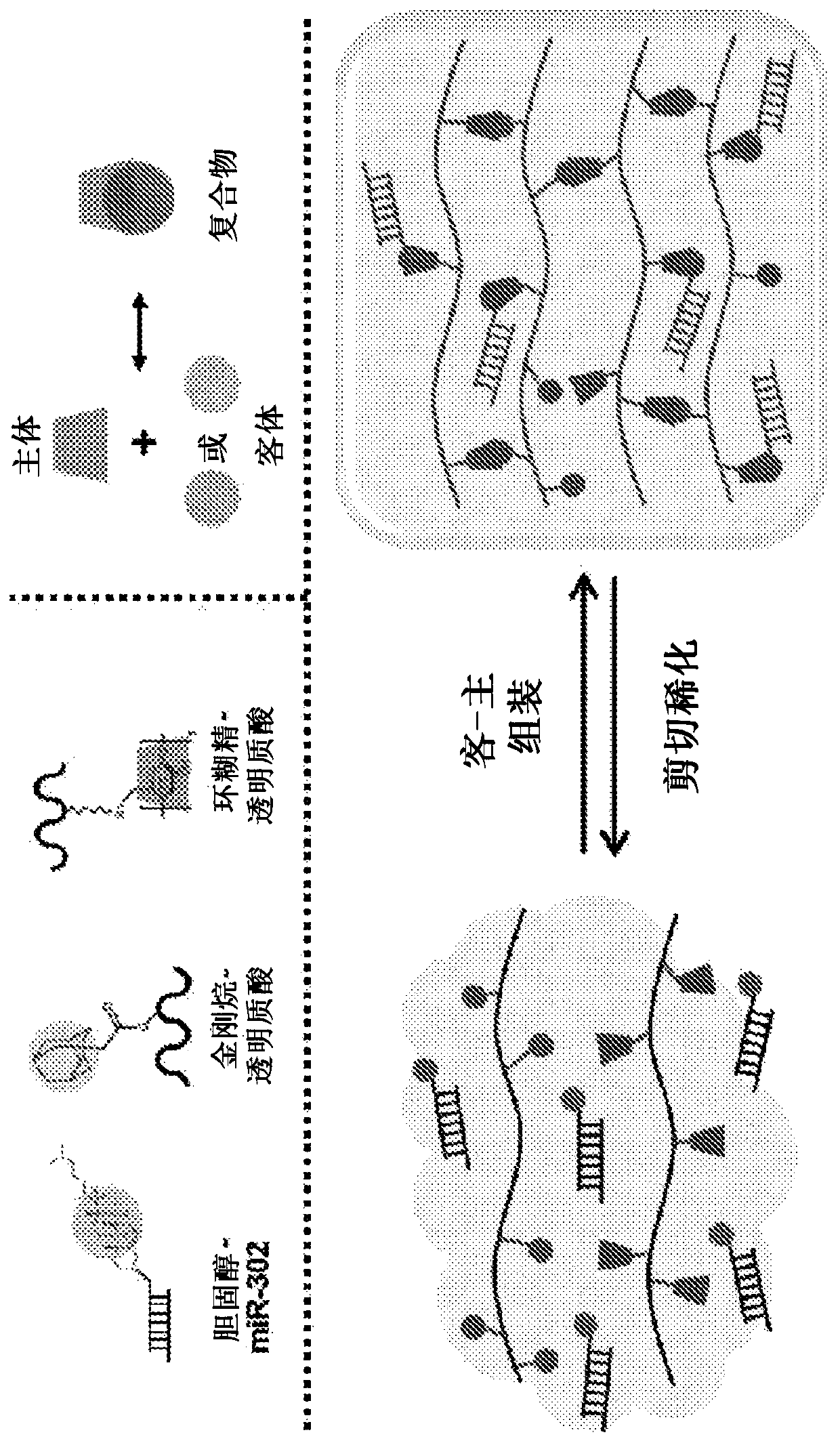
[0078] Preparation and loading of host-guest hydrogels
[0079] Materials Synthesis: Sodium hyaluronate (LifeCore, Chaska, MN) was converted to tetrabutylammonium salt (HA-TBA) by exchange with Dowex-100 resin and neutralization with tetrabutylammonium hydroxide. CD-HA and AD-HA were synthesized as previously described. Briefly, 6-(6-aminohexyl)amino-6-deoxy - Amidation reaction between cyclodextrin and HA-TBA to prepare CD-HA. AD-HA is prepared by combining HA-TBA with 1-adamantaneacetic acid in di-tert-butyl bicarbonate (BOC 2 O) and 4-dimethylaminopyridine (DMAP) in the esterification and synthesis. The product was dialyzed, frozen and lyophilized before use. Use 360MHz 1 H NMR (Bruker) determined the modification of the final product, for CD-HA and AD-HA, the HA modification was about 25% HA disaccharide.
[0080] The miRNA molecules used have the following sequences:
[0081] cel-miR-67 (miR-NC)
[0082] 5'-CGCUCAUUCUGCCGGUUGUUAUG-3' (guide; SEQ ID NO: 14)
[008...
Embodiment 2
[0091] Rhodamine Quenching Test
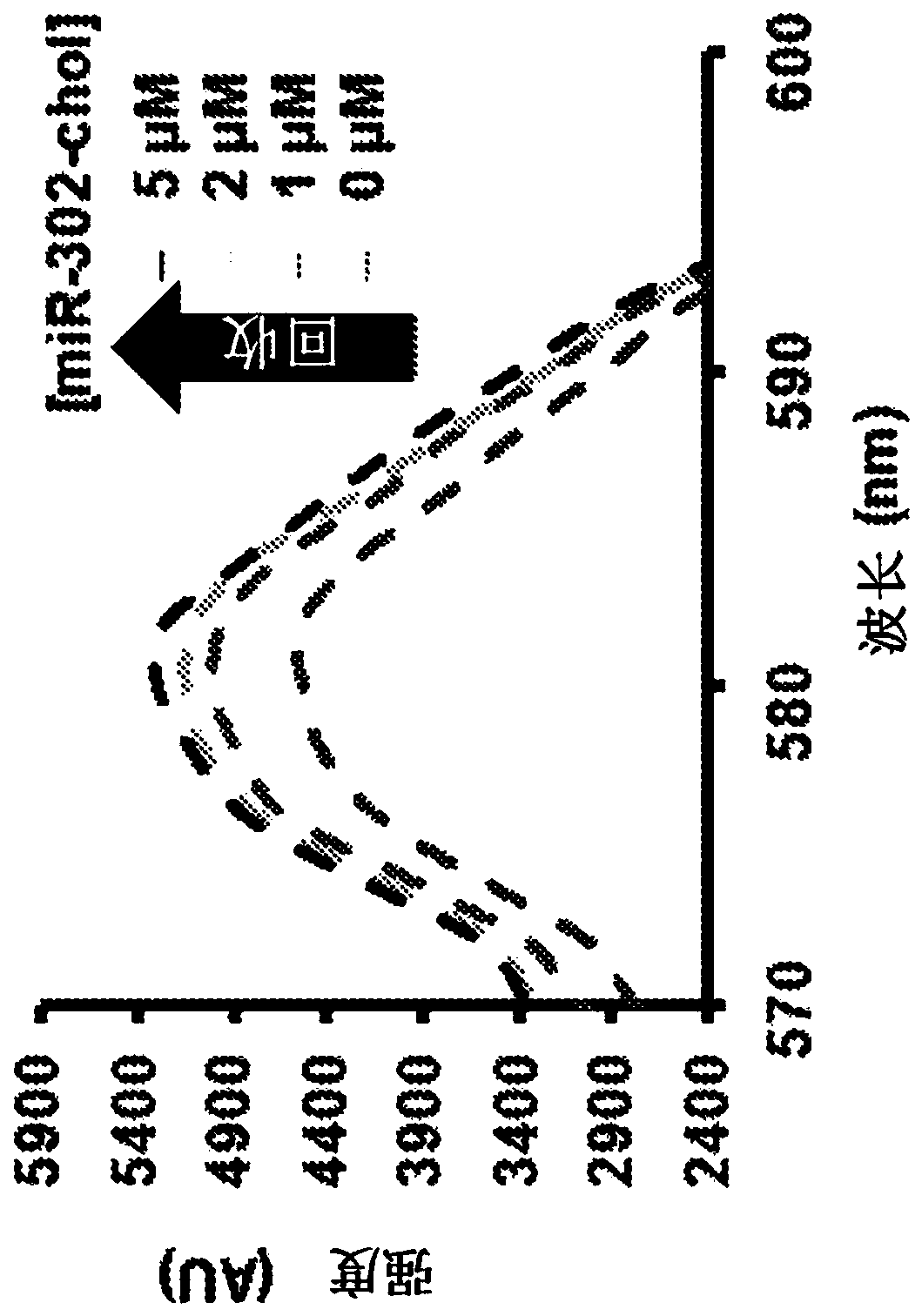
[0092] To further examine the interaction between cholesterol and CD, we developed a fluorescent binding assay to measure the interaction between cholesterol-modified miR302b / c and CD-HA. The assay is based on the ability of cholesterol to bind to and displace rhodamine B from β-cyclodextrin, resulting in quenching and fluorescence enhancement. Gels were prepared as described in Example 1. Rhodamine B (50 ng / μL) was mixed with varying amounts of CD-HA (0-50 ng / μL) in 200 μL of DI HO to a final concentration to determine the saturation concentration for quenching. For non-quenching assays, rhodamine B (50 g / μL) was mixed with miR-302 mimic (0-5 μM) in a final volume of 200 μL. Emission from 530 to 580 nm was measured on a Tecan Infinite200 plate reader with excitation at 550 nm. The miR-302b-chol affinity to the Rho / CD-HA complex was calculated by fitting the Benesi-Hildebrand equation.
[0093] We found that cholesterol-modified miR-302 mi...
Embodiment 3
[0095] Rheological properties
[0096] To confirm that cholesterol-modified miR-302b and miR-302c mimics (hereafter referred to as miR-302) did not affect the mechanical and erosion behaviors of hydrogels, we performed oscillatory rheological and hydrogel corrosion tests.
[0097]Measurements were performed using an AR2000 stress-controlled rheometer (TA Instruments) equipped with a 20 mm diameter cone-plate geometry, a 42 s cone angle for 59 min and a gap of 27 μm. The rheological properties were checked by time sweep (1.0 Hz; 0.5% strain). For shear recovery experiments, shear thinning was performed at 250% strain and recovery at 0.5% strain, each at 20 Hz.
[0098] Inclusion of miR-302 in the system also did not affect gel erosion at two weeks by the uronic acid assay measuring total hyaluronan degradation (Fig. 1E).
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscoelasticity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com