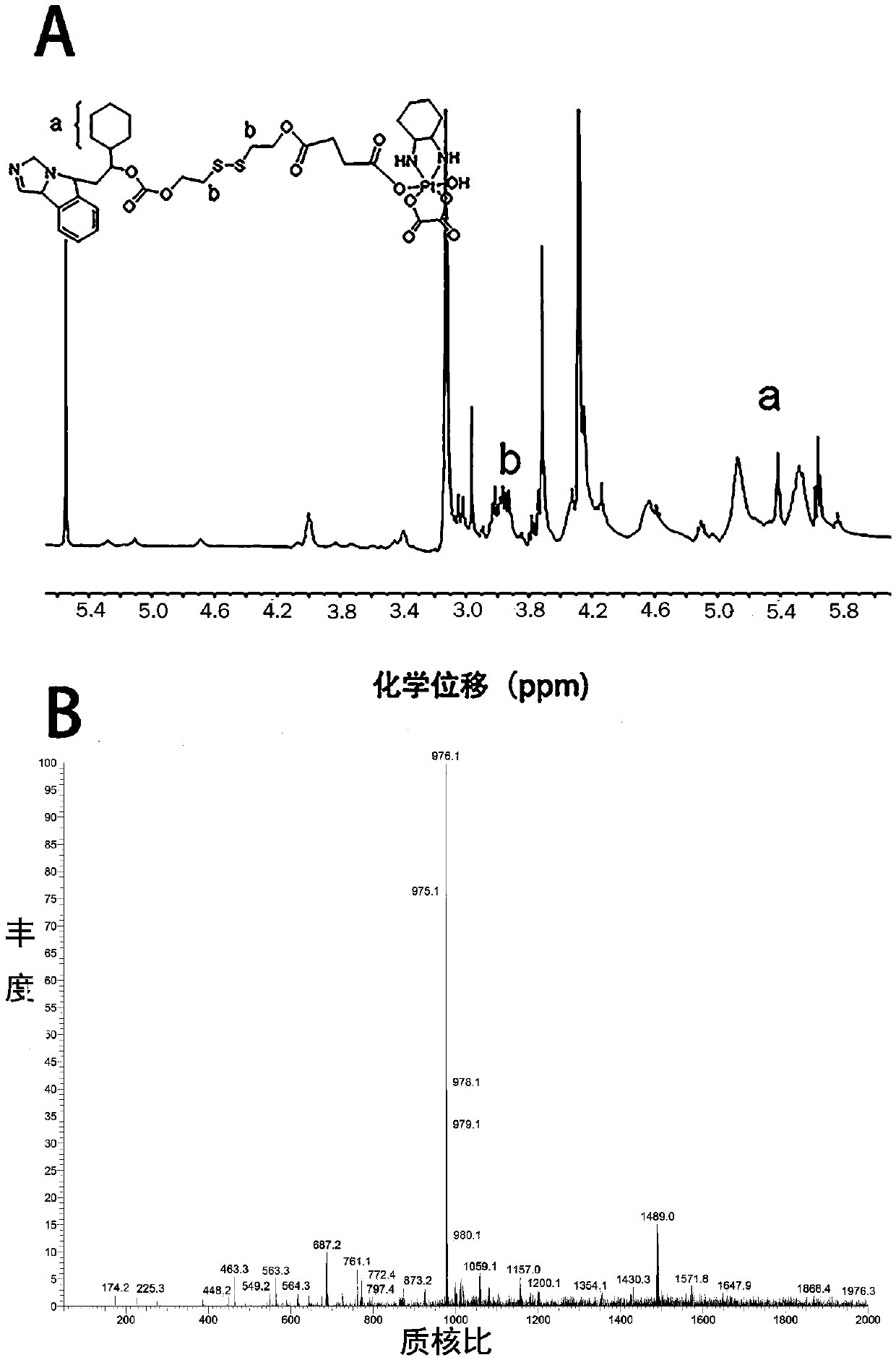
Oxaliplatin conjugated prodrug, preparation method and uses thereof
A technology of oxaliplatin and prodrug, applied in the field of oxaliplatin conjugated prodrug, can solve problems such as T cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1. Preparation of oxidized oxaliplatin
[0054] Weigh 500mg of oxaliplatin, suspend it in 30ml of water, add 8ml of 30% hydrogen peroxide, put it in a 50ml round bottom flask, stir and react in the dark at 40°C for 10 hours, remove the solvent by rotary evaporation, and dry in vacuo to obtain oxaliplatin Platinum.
Embodiment 2
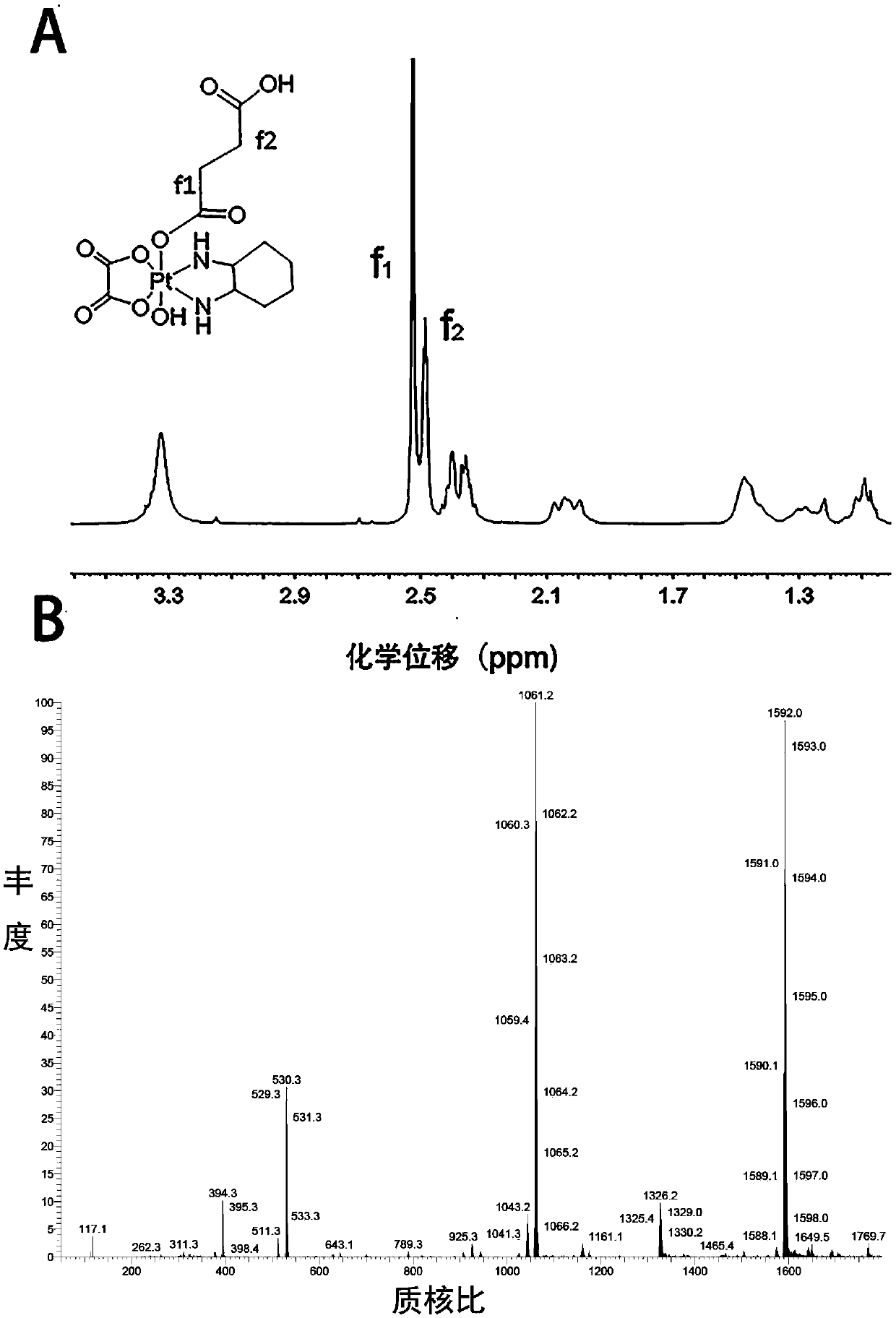
[0055] Example 2. Preparation of Monocarboxylated Oxaliplatin
[0056] Take 413 mg of oxidized oxaliplatin prepared in Example 1, dissolve it in 5 ml of anhydrous dimethyl sulfoxide, add 100 mg of succinic anhydride, react at room temperature for 12 hours, precipitate with ether, and dry in vacuo to obtain monocarboxylated oxaliplatin Platinum. The resulting material was characterized by proton nuclear magnetic resonance and mass spectrometry, and the results were as follows: figure 1 shown.
Embodiment 3
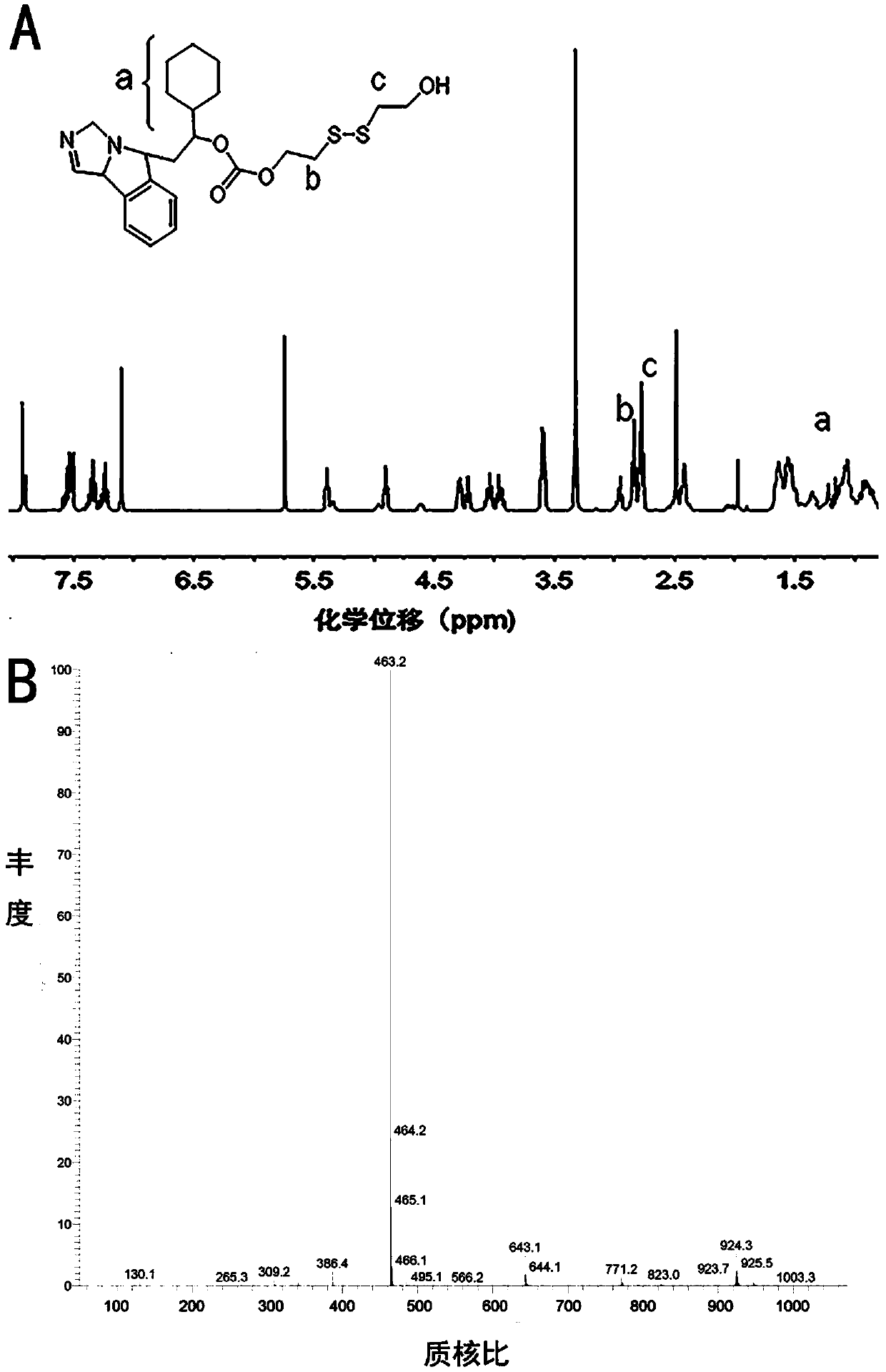
[0057] The preparation of the disulfide of embodiment 3.NLG919
[0058] Weigh 300mg of NLG919, 450mg of DMAP dissolved in 15ml of DCM in a 200ml round bottom flask. Weigh 140mg of triphosgene and dissolve it in 5ml of DCM, and slowly add it dropwise into the round bottom flask under stirring. After reacting for 30min, slowly add 500ml of bis(2-hydroxyethyl) disulfide into the reaction system dropwise. After reacting at room temperature for 20 h, the product was spin-dried and vacuum-dried to obtain the disulfide of NLG919. The resulting material was characterized by proton nuclear magnetic resonance and mass spectrometry, and the results were as follows: figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com