Method of conducting hydroformylation reaction on C4 mixture to prepare aldehydes
A technology of tetrahydroformyl and mixed carbon 4, applied in the direction of carbon monoxide reaction preparation, chemical recovery, organic chemistry, etc., can solve problems such as large differences and achieve the effect of avoiding separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
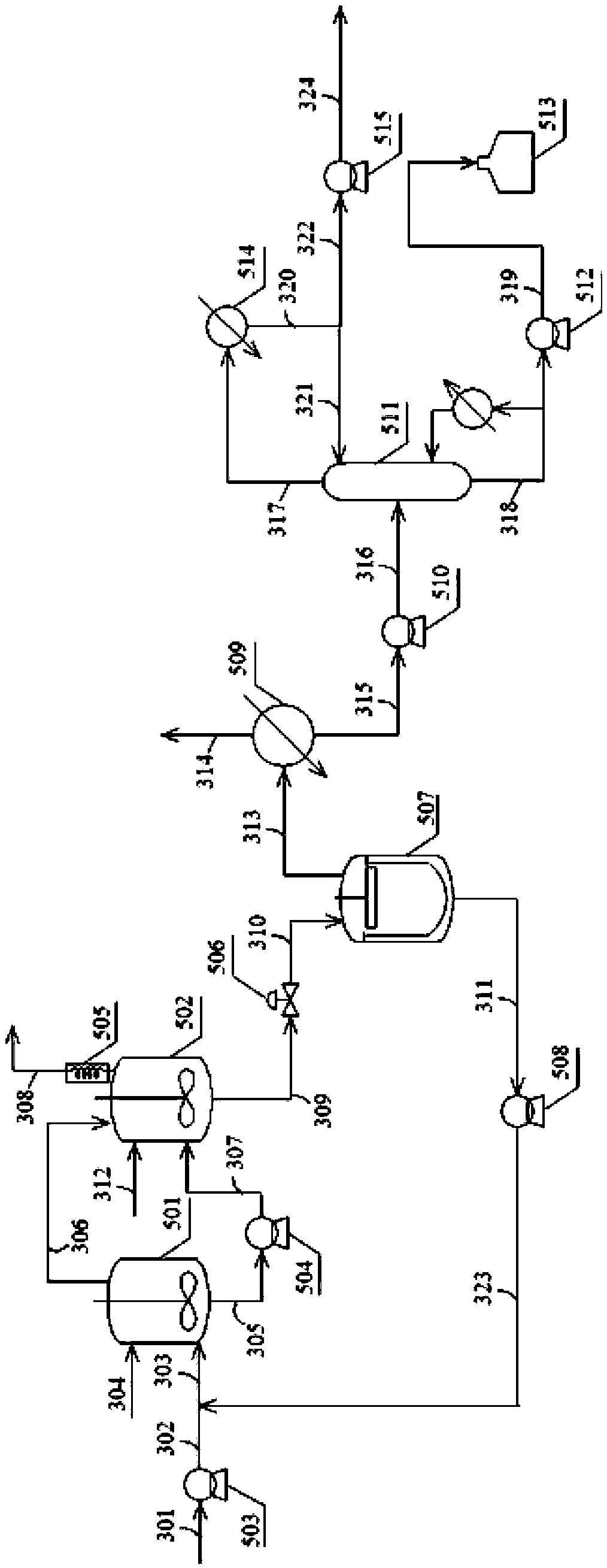
[0041] The reaction of embodiment 1 is in such as figure 2 and image 3 In the setup shown:
[0042] The first reaction zone consists of two stirred tanks connected in series, and the intermediate separation zone consists of a thin film evaporator and a packed tower. The first catalyst used in the first reaction zone is a rhodium-triphenylphosphine complex catalyst generated in situ by a rhodium compound and triphenylphosphine, the rhodium compound is rhodium acetylacetonate dicarbonyl, and the solvent for dissolving the first catalyst is butyl aldehyde.
[0043] from storage tank ( figure 2 Not shown) mixed butene (composition is 1-butene 36.2wt%, trans-2-butene 0.1wt%, isobutene 55.2wt%, isobutane 8.0wt%, n-butane 0.5wt%) with flow 200g / hr is sent into the pipeline 302 by the pump 503 through the pipeline 301, mixed with the discharge 323 from the bottom of the thin film evaporator 507, and then sent to the bottom of the reactor 501 through the pipeline 303 by the bott...
Embodiment 2
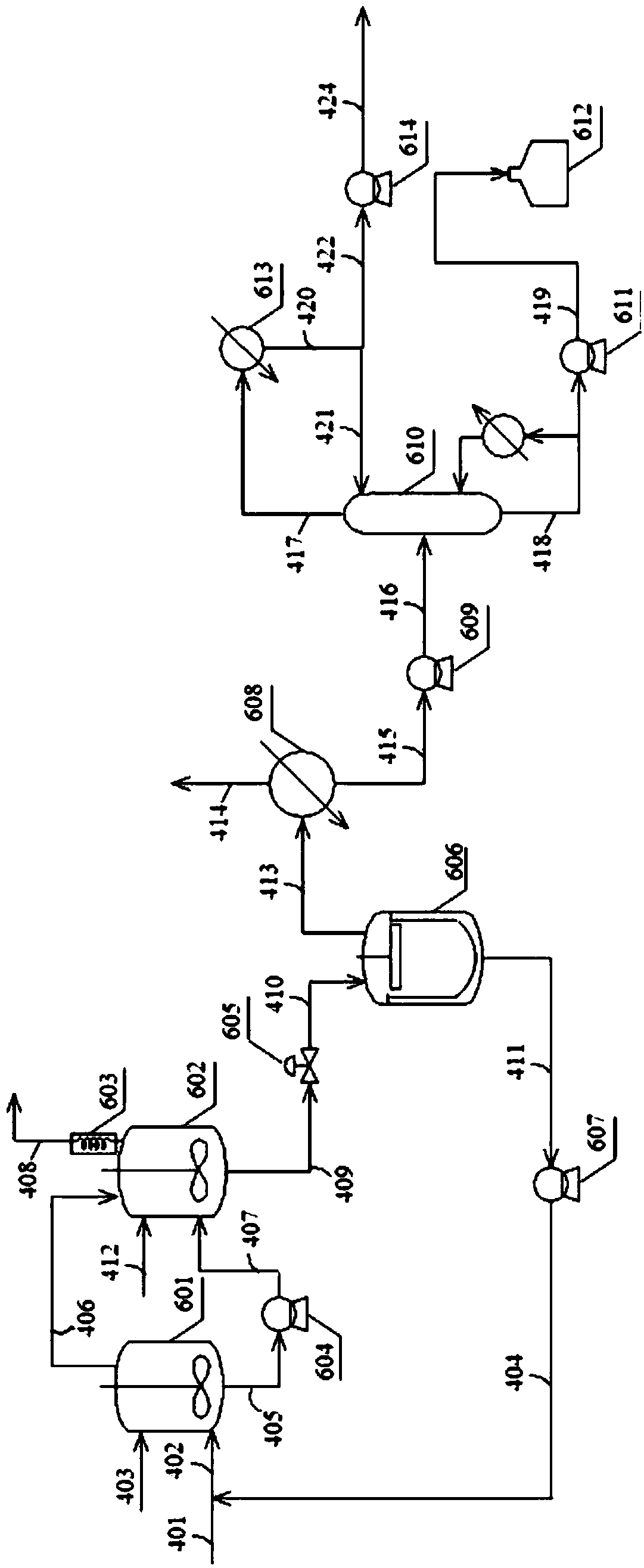
[0053] Embodiment 2 adopts the technique identical with embodiment 1, and the condition of the first reaction zone and intermediate separation zone is identical with embodiment 1, and the difference among embodiment 2 and embodiment 1 is:
[0054] The second catalyst used in the second reaction zone is a rhodium-phosphonite catalyst generated in situ from a rhodium compound and a phosphite, the rhodium compound is rhodium acetylacetonate dicarbonyl, and the phosphonite ligand is 6,6'- [(3,3',5,5'-tetra-tert-butyl-1-1'-biphenyl)2,2'-dioxo]-bis-bibenzo[d,f][1,3,2 ] dioxaphosphapine ring, the solvent used to dissolve the second catalyst is butyraldehyde. In the reactor 601, the rhodium concentration is 200ppm, and the phosphonite ligand concentration is 0.6wt%; in the reactor 602, the rhodium concentration is 200ppm, and the phosphonite ligand concentration is 0.6wt%. The total pressure of the reactor 601 is 1.3MPa, and the temperature is 80°C; the total pressure of the reactor ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com