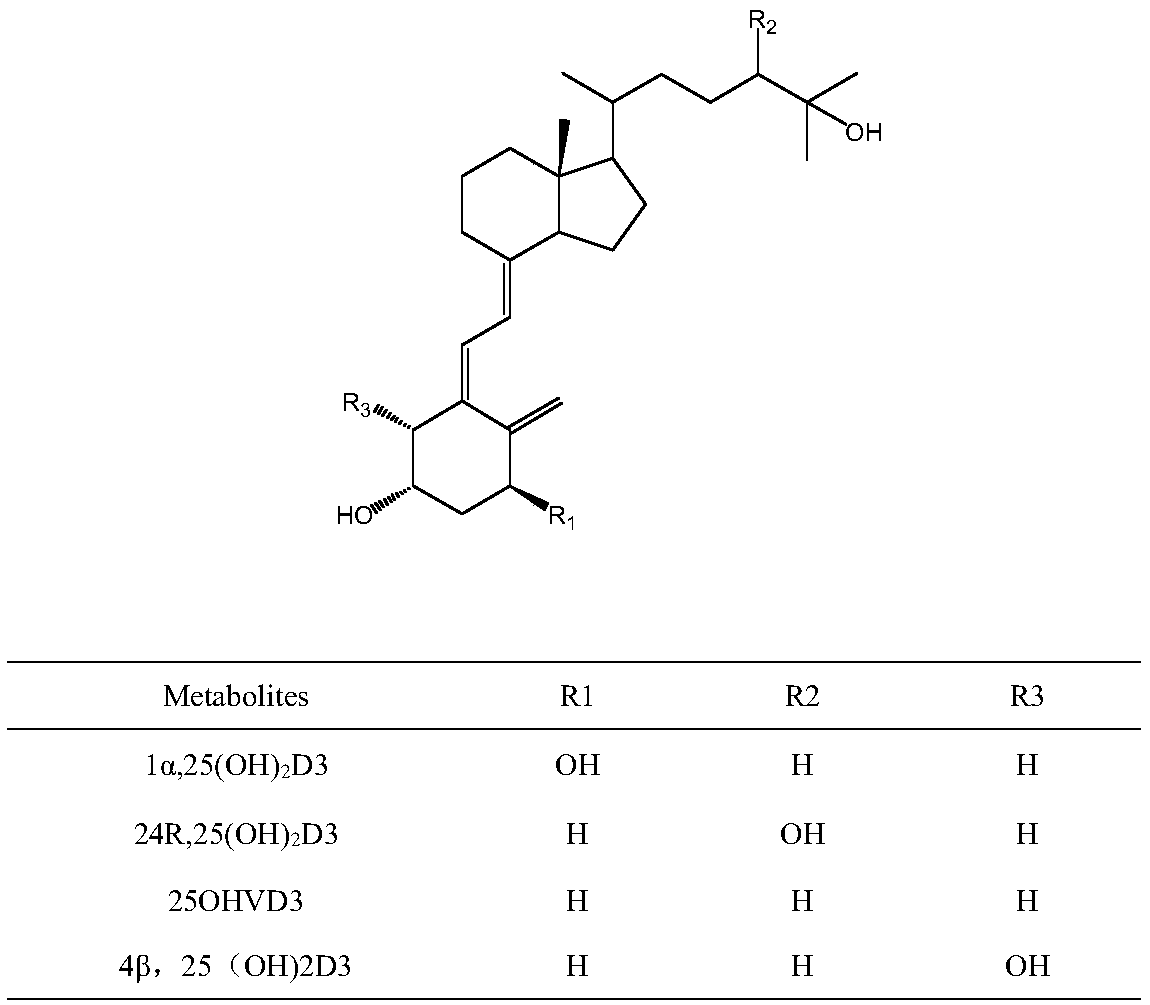
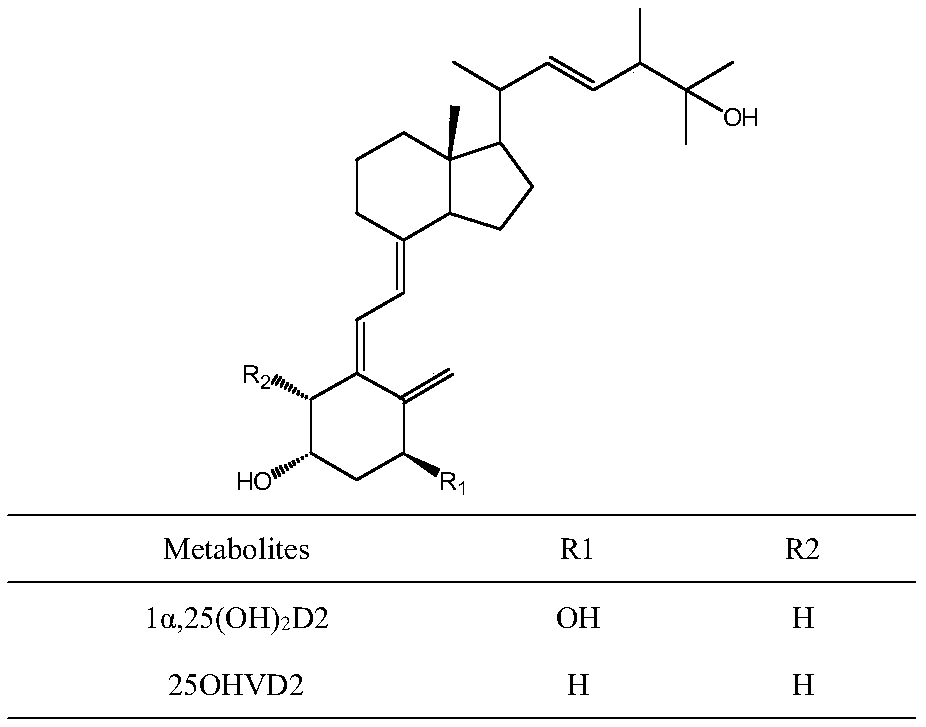
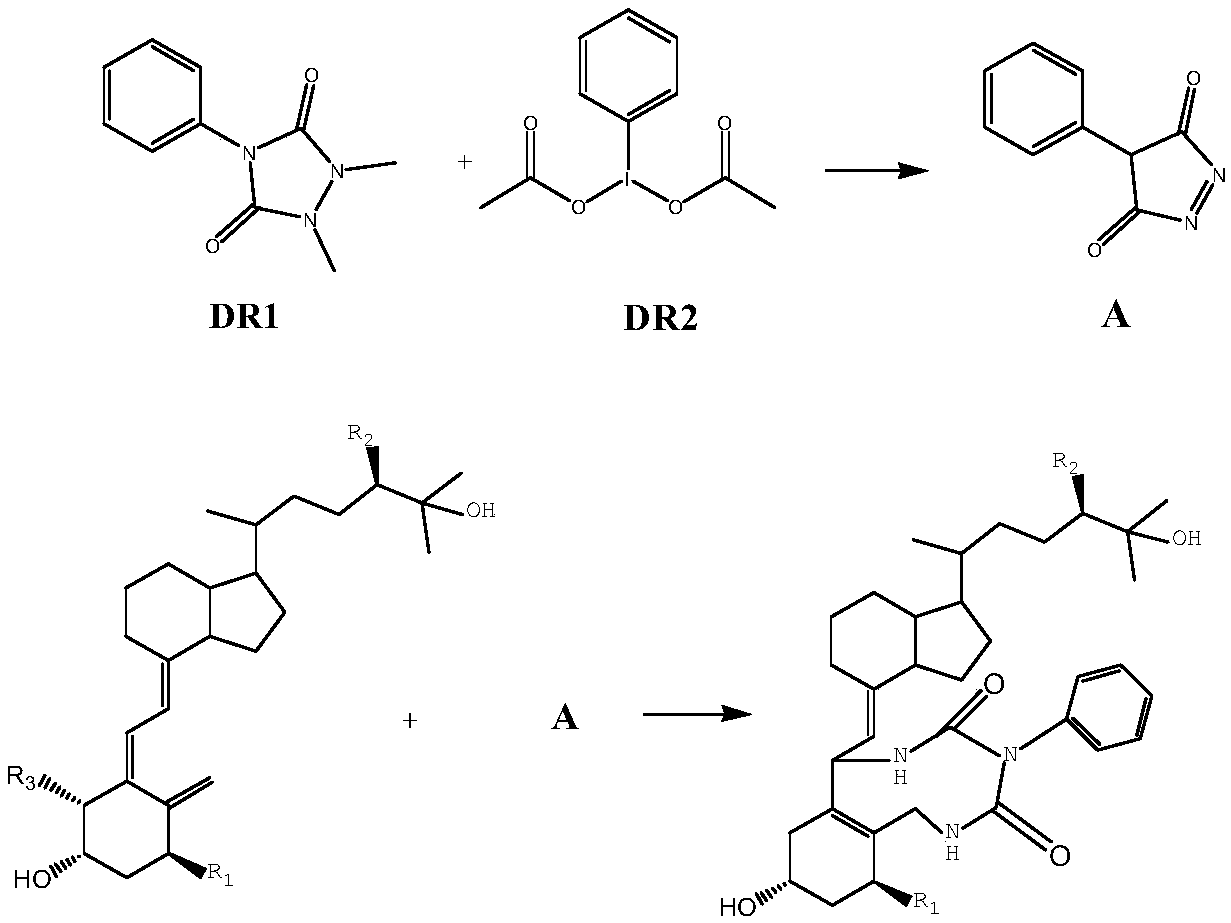
Method for detecting vitamin D metabolite
A detection method and metabolite technology, applied in the field of analysis and detection, can solve the problems of large sample size, difficulty in obtaining such substrates, inconvenient transportation, etc., to eliminate detection interference, improve derivatization efficiency, and improve derivatization speed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The addition order of embodiment 1 derivative and the selection optimization of substituting matrix
[0031] 1.1. The solution of innovative chemical derivatization to the sensitivity, speed and cost of the method:
[0032] See the principle of innovative chemical derivatization reaction image 3 .Derivatization reagents DR1 and DR2 react to produce product A, and product A and vitamin D metabolites undergo the next step of derivatization reaction of Diels-Alder addition reaction (Diels-Alder reaction).
[0033] According to attached Figure 4A , it can be found that without derivatization, 1α,25-dihydroxyvitamin D3 in serum cannot be detected even under the instrument conditions of the high-end instrument ABQTRAP 5500 in current clinical research. After derivatization, the target components of the same samples were accurately detected ( Figure 4B ). The reason is that although the derivatization reagent used in the present invention is still PTAD in essence, it is g...
Embodiment 2
[0045] Example 2 Detection method of 1α, 25-dihydroxyvitamin D3
[0046] Taking the development process of the method of α,25-dihydroxyvitamin D3 in Example 1 as an example, the reagents and instrument information used in the method development are described in detail as follows. Other metabolites are applicable in principle, and the specific parameters should be determined by those skilled in the art according to the target of metabolite detection, through experiments to determine the mass spectrometry detection conditions and liquid phase separation conditions, and the derivatization conditions basically do not need to be adjusted.
[0047] 2.1. Reagent and instrument information:
[0048] 2.1.1 Reference compounds:
[0049] 1α, 25-dihydroxyvitamin D3 and 1α, 25-dihydroxyvitamin D3-d3 were purchased from Isosciences, and 1 mL of ethanol solution was placed in an ampoule.
[0050] 2.1.2 Reagents:
[0051] Methyl tert-butyl ether (HPLC grade), methanol (HPLC grade), ammoniu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com