Carbamate compound, pharmaceutical composition and application of carbamate compound
A technology of carbamates and compounds, applied in the field of medicine, can solve problems such as poor activity of TRK inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
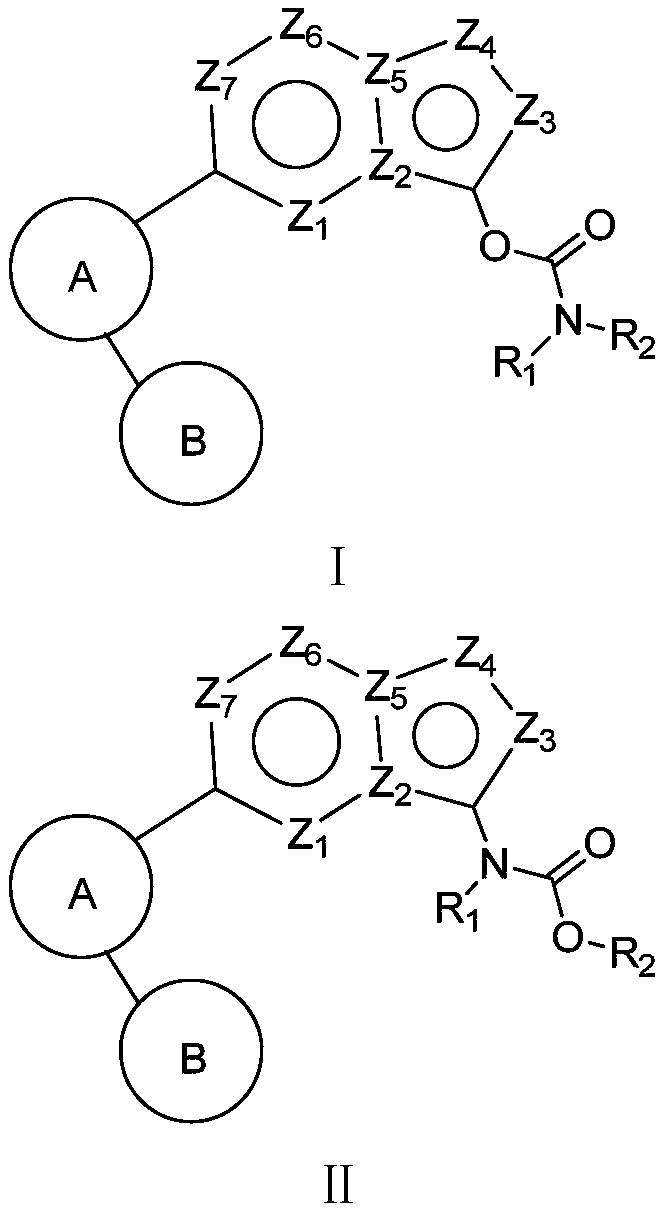
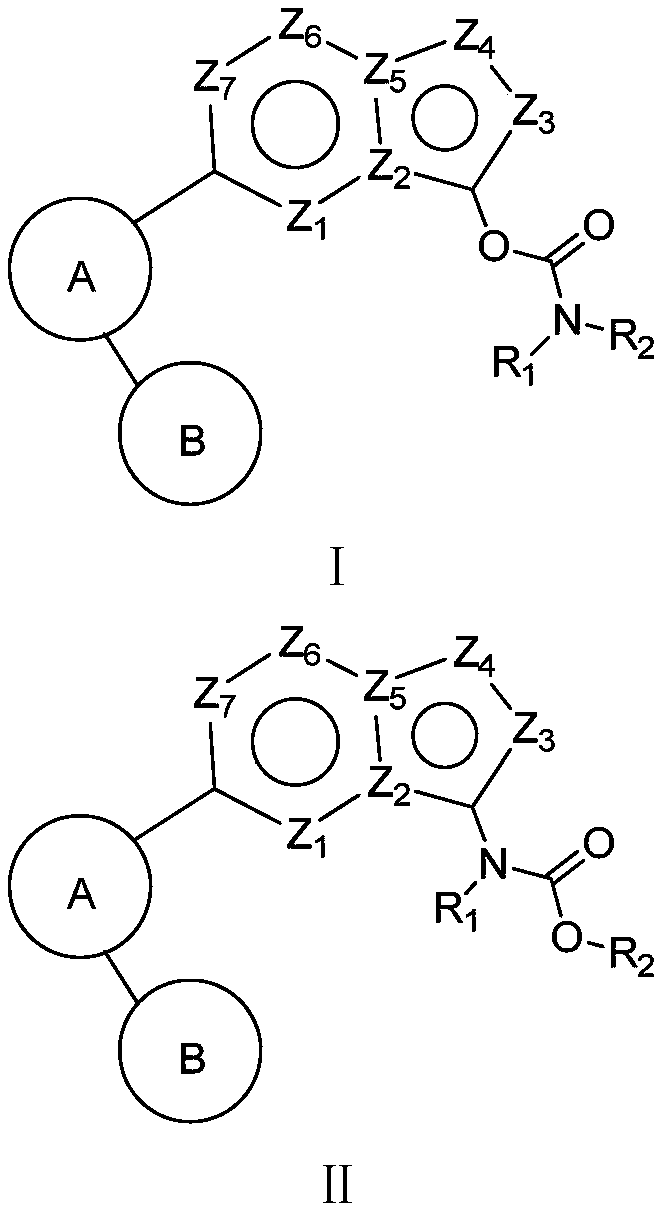
[0047] The carbamate compound of present embodiment, its structural formula is as follows:
[0048]
[0049] The specific process route is as follows:
[0050]
[0051] Concrete preparation method comprises the following steps:
[0052] (1) Compound 1 (20.0g, 0.13mol, 1eq) was dissolved in dry 1,2-dichloroethane (EDC) (200ml), and AlCl was added under ice-cooling 3 (26.1g, 0.195mol, 1.5eq) and acetyl chloride (15.3g, 0.195mol, 1.5eq), then heated to 80°C for 12h to obtain a reaction solution; slowly added the reaction solution to ice water, added ethyl acetate for extraction, Washed with brine three times, combined the organic phases, dried over anhydrous sodium sulfate, spin-dried the organic phases, purified by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain compound 2 (5.6g white solid, yield 23.5%) ;
[0053] (2) Dissolve compound 2a (2.0g, 10.9mmol, 1eq) in ethanol (EtOH) (20mL), then add compound 2 (2.56g, 13.1mmol, 1.2eq) and triethylamin...
Embodiment 2
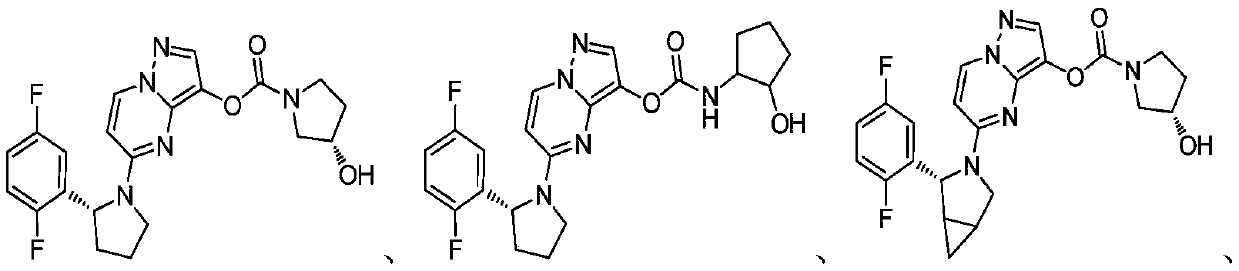
[0060] The carbamate compound of present embodiment, its structural formula is as follows:
[0061]
[0062] The nuclear magnetic resonance spectrum of this carbamate compound ( 1 H NMR, 400MHz) features: with CDCl 3 is the solvent, where the peaks are assigned as: δ8.08(s,1H),7.88(s,1H),7.04(m,1H),6.92(m,1H),6.74(m,1H),5.82(s, 1H), 5.41(s,1H), 4.13(m,1H), 7.70-3.94(m,3H), 0.70-2.47(m,10H); LCMS: M+1:444.
Embodiment 3
[0064] The carbamate compound of present embodiment, its structural formula is as follows:
[0065]
[0066] The nuclear magnetic resonance spectrum of this carbamate compound ( 1 H NMR, 400MHz) features: with CDCl 3 As a solvent, the peaks are assigned as: δ8.07(d, J=7.8Hz, 1H), 7.90(s, 1H), 7.04(m, 1H), 6.89(m, 1H), 6.66(m, 1H) ,5.90(s,1H),5.41(d,J=4.7Hz,1H),4.56(s,1H),4.01(d,J=10.7Hz,1H),3.92(m,1H),3.78–3.45( m,4H),2.20(m,1H),2.04(m,2H),1.87(m,1H),1.73(m,1H),0.66(m,1H),0.41(m,1H); LCMS: M +1:442.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com