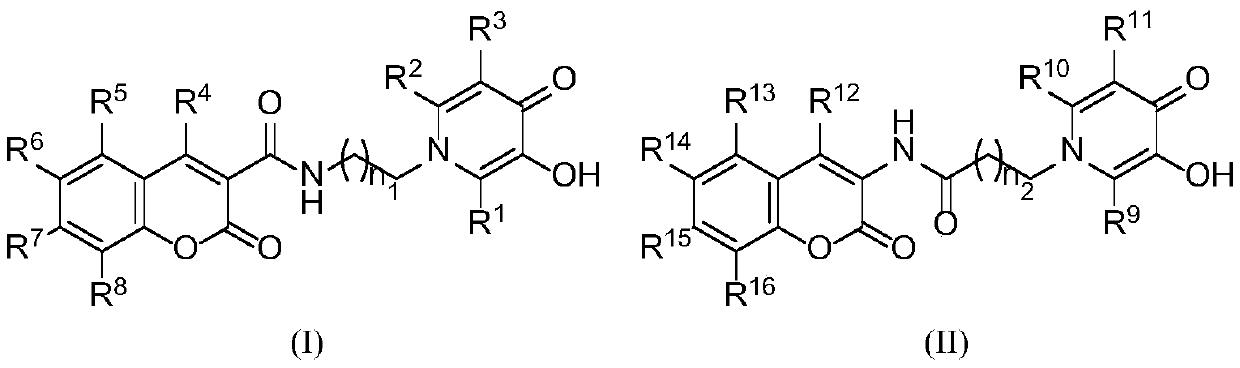
Coumarin hybrid pyridinone amide derivative with potential anti-AD activity and preparation method and application of derivative
A technology of coumarin and pyridone, applied in the direction of anti-toxins, drug combinations, nervous system diseases, etc., can solve the problems of vague pathogenic mechanism, low pertinence, and incomplete cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
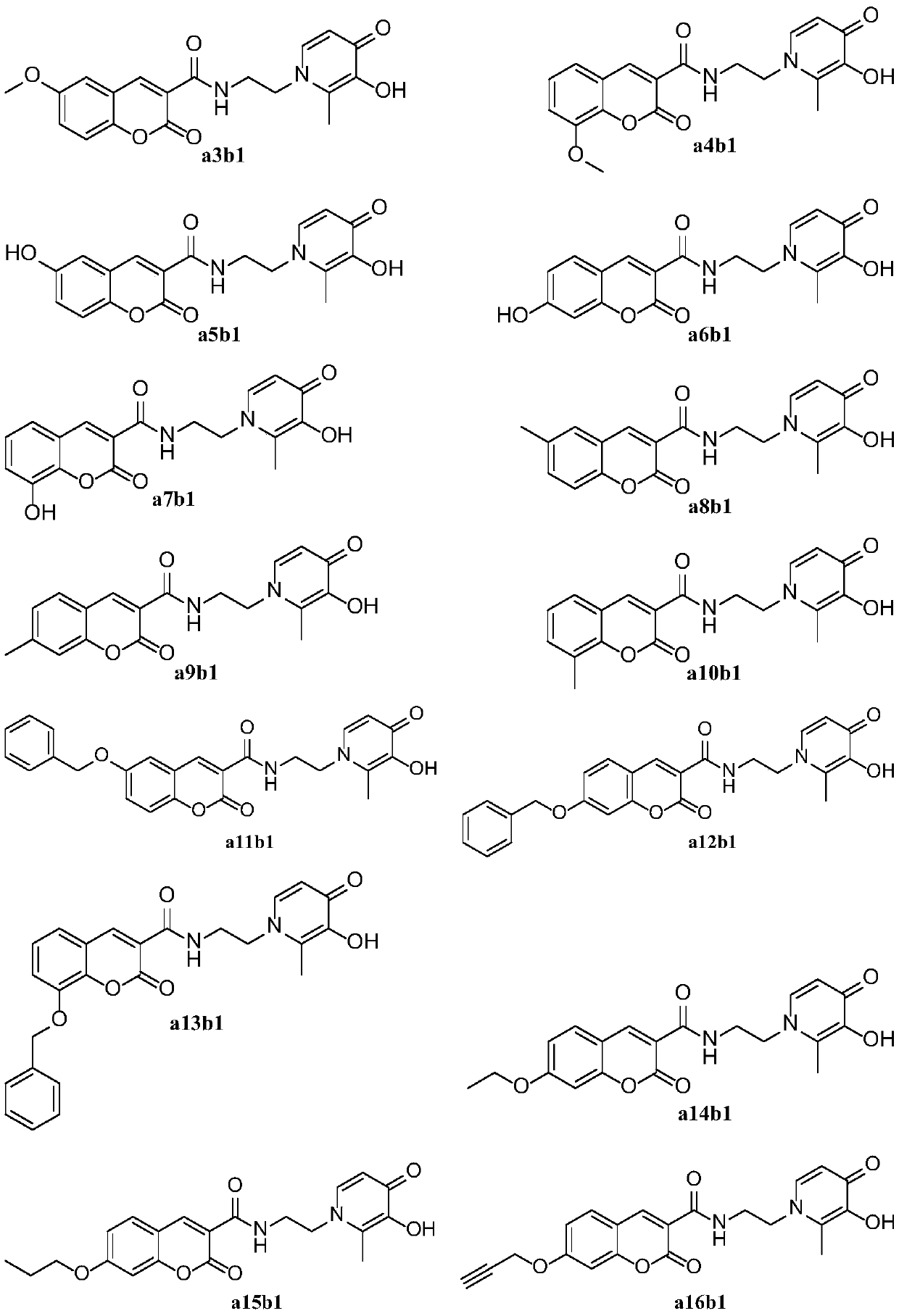
Image
Examples
Embodiment 1
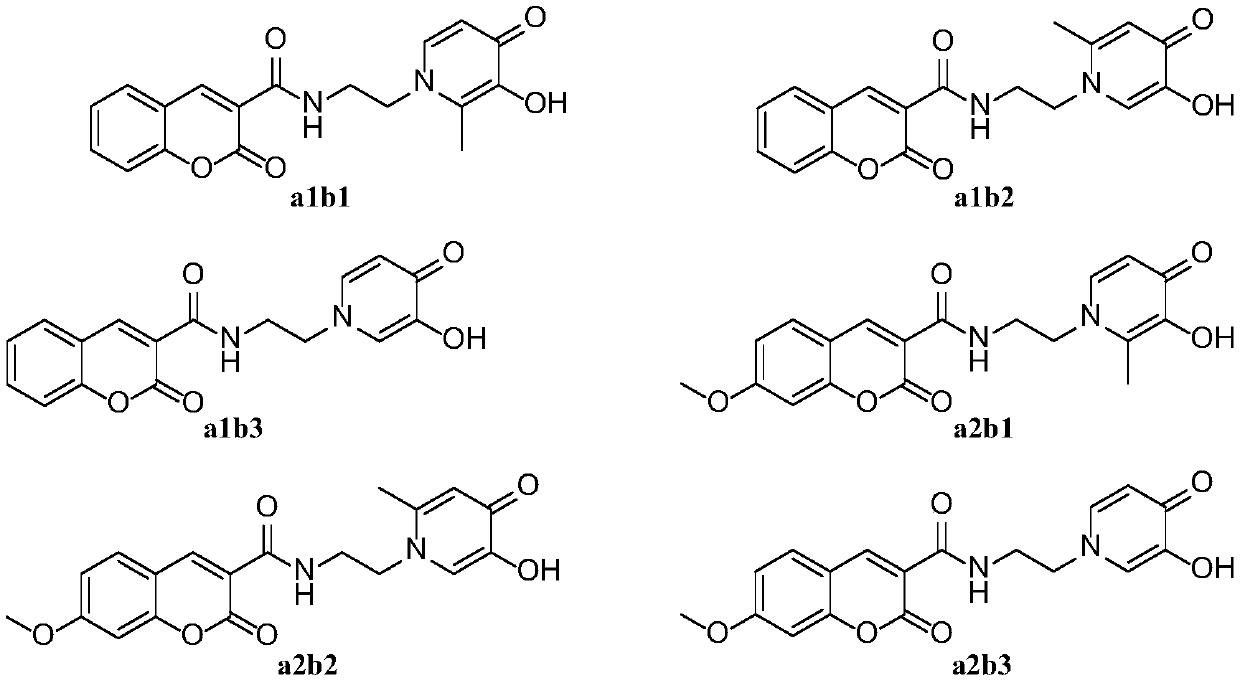
[0063] N-(2-(2-methyl-3-hydroxy-1(4H)-4-oxopyridyl)ethyl)-2-oxo-2H-chromene-3-carboxamide (a1b1) preparation method
[0064]Add maltol (7.56g, 60mmol), methyl iodide (25.56g, 180mmol), anhydrous potassium carbonate (24.84g, 180mmol), acetone (100mL) into a 250mL single-necked bottle, heat and reflux for 3h, monitor the reaction by TLC, and wait for the raw material After the conversion is complete, stop the reaction, concentrate the reaction solution to obtain a solid, add 100mL water to dissolve, extract with 100mL×4 dichloromethane, combine the organic layers, dry the organic layer with anhydrous sodium sulfate, and concentrate to obtain a yellow oily liquid 2-methyl- 3-methoxypyran-4-one (8.35 g), yield 99.4%.
[0065] Pyrone (2.80g, 20mmol), ethylenediamine (1.26g, 21mmol), sodium hydroxide (0.72g, 18mmol), ethanol (20mL), water (18mL), and Heat at reflux at 70°C for 1.5h. After the reaction, concentrate the reaction solution and purify it by silica gel column chromatogr...
Embodiment 2
[0074] N-(2-(6-methyl-3-hydroxy-1(4H)-4-oxopyridyl)ethyl)-2-oxo-2H-chromene-3-carboxamide (a1b2) preparation method
[0075] Add kojic acid (21.3g, 150mmol) and thionyl chloride (85mL) into a 250mL single-necked bottle, connect the reaction bottle to a gas absorption device, and stir at room temperature for 2h. After the reaction, suction filtration was performed, and the filter cake was washed with petroleum ether until the filtrate was colorless. The filter cake was recrystallized with water to obtain a white solid (21.7 g), with a yield of 90.3%. Add the above-mentioned white solid (21.7g, 135.5mmol) and distilled water (100mL) into a 500mL two-necked flask, heat up to 50°C, then add zinc powder (17.6g, 271mmol), measure concentrated hydrochloric acid (40.9mL) and place in constant In the pressure dropping funnel, slowly add dropwise, control the temperature between 70-80°C, after dropping, control the temperature at 75°C for 4 hours. After the reaction, the reaction solu...
Embodiment 3
[0086] The preparation method of N-(2-(3-hydroxyl-1(4H)-4-oxopyridyl)ethyl)-2-oxo-2H-benzopyran-3-carboxamide (a1b3)
[0087]Kojic acid (14.2g, 100mmol), methyl iodide (42.6g, 300mmol), anhydrous potassium carbonate (41.4g, 300mmol) and acetone (100mL) were added to a 100mL single-necked bottle, and the reaction was refluxed for 3h. After the reaction, concentrate the reaction solution to obtain a solid, add 150mL of water, extract with 150mL×3 methylene chloride, combine the organic layers, wash with 150mL×2 saturated brine, dry over anhydrous sodium sulfate, and concentrate to obtain a yellow solid (14.35g ), yield 92%.
[0088] Add the above yellow solid (6.24g, 40mmol) and acetone (600mL) into a 1000mL four-necked flask, stir mechanically, and heat up to reflux. Put Jones reagent (45mL) in a constant pressure dropping funnel, add it dropwise slowly, after dropping, reflux reaction for 40min, monitor the reaction by TLC, stop the reaction after the conversion of the raw ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com