1,3-disubstituted indoline derivative and preparation method thereof
A technology for indoline and derivatives, applied in the field of indoline derivatives and their preparation, can solve problems such as high temperature, harsh reaction conditions, and long reaction time, and achieve the effects of simple raw materials, fast reaction speed, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
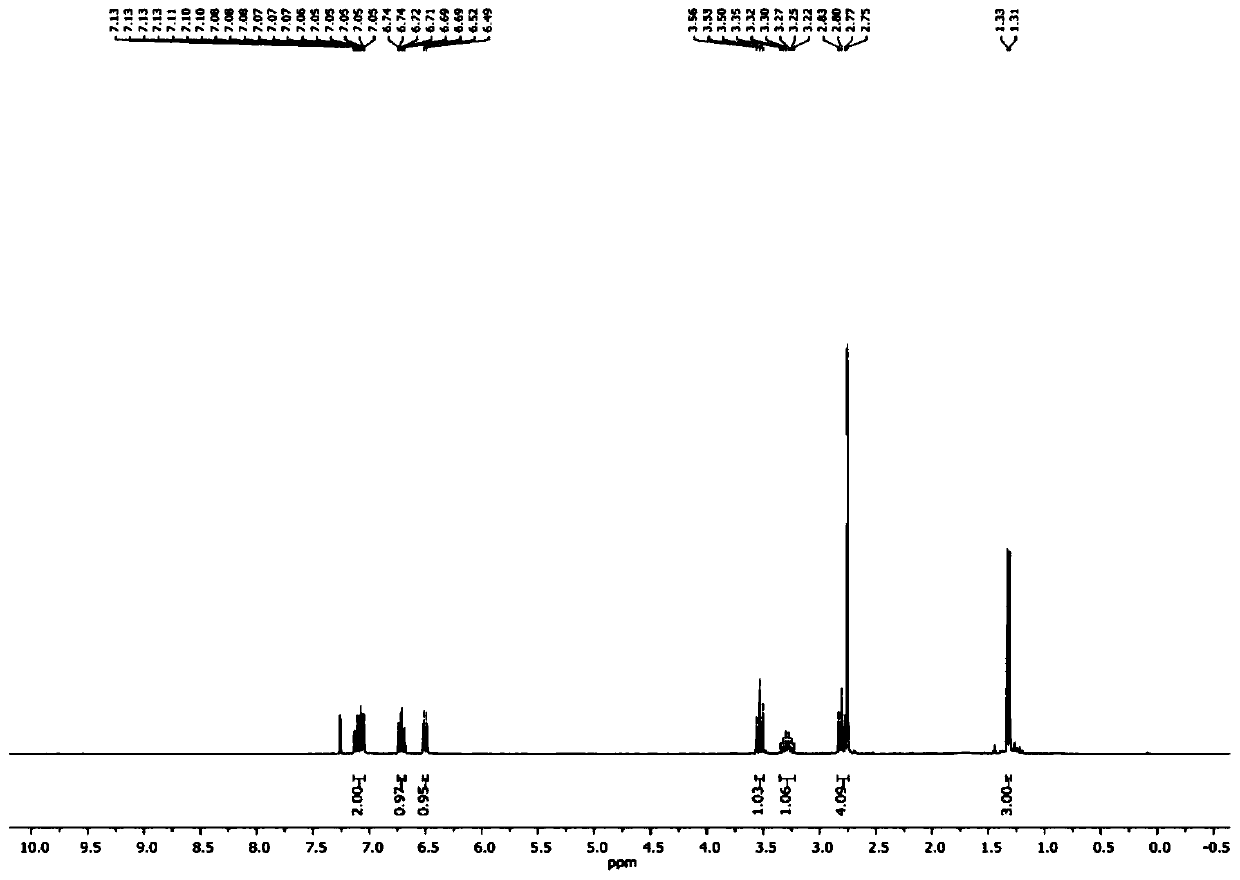
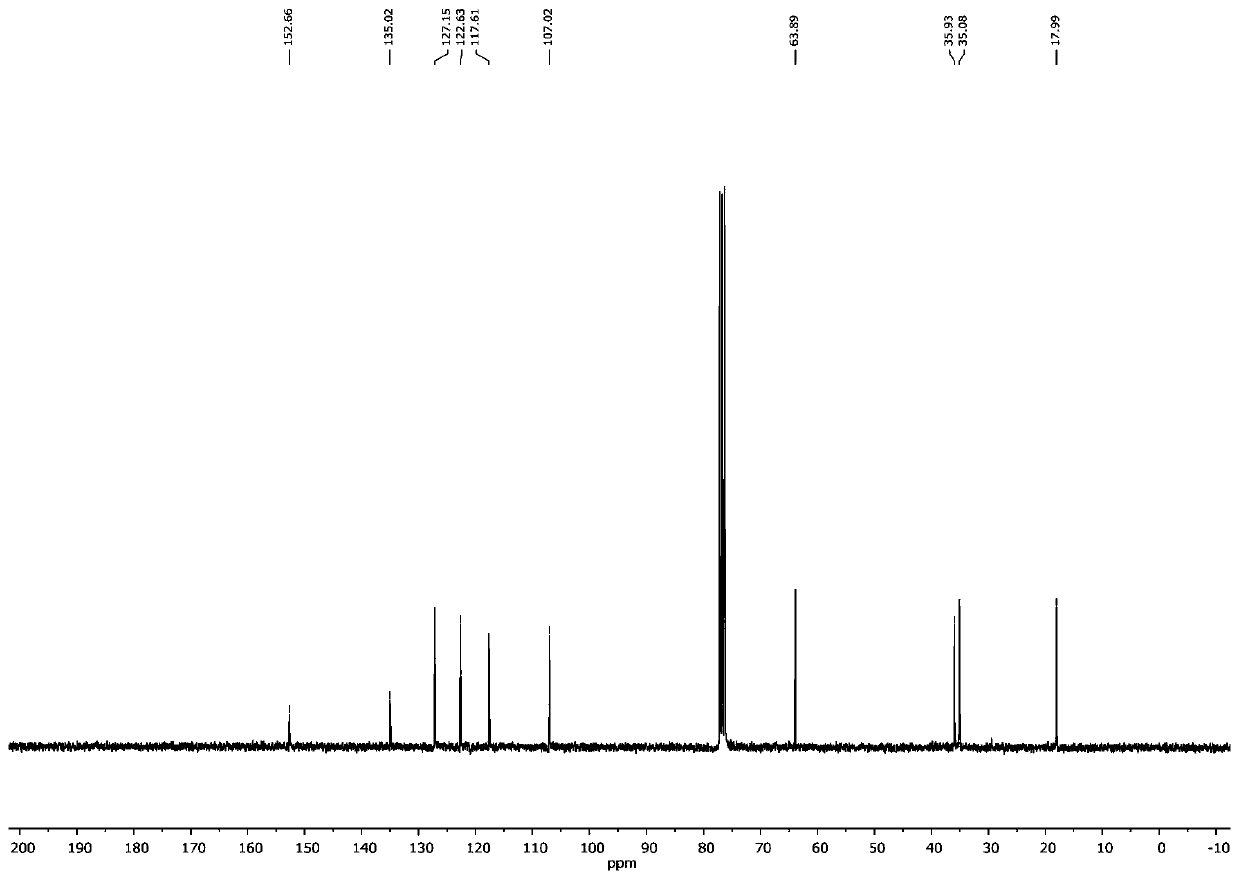
[0072] Example 1: Synthesis of 1,3-dimethylindoline from 2-iodoaniline
[0073]
[0074] 2-iodoaniline (2.19 g), potassium carbonate (2.76 g), dimethyl sulfoxide (DMSO, 50 ml) were added to a round bottom flask, and methyl iodide (CH 3 1, 1.42 grams), the dropwise addition was completed, and the ice bath was removed, and the resulting mixed system was warmed up to room temperature, and continued to stir for 12 hours; afterward, continue to drip allyl bromide (1.21 grams) under the ice bath, after the dropwise addition, room temperature reaction After 16 hours, add deionized water (50 ml) to quench the reaction, use 50 ml of ether to extract 3 times, combine the organic phases, and remove the solvent under reduced pressure on a rotary evaporator to obtain N-methyl-N-allyl- 2-iodoaniline crude product (2.42 grams), this product can carry out next step reaction without further purification;
[0075] SmI 2 (0.1 mol / L tetrahydrofuran solution, 120 ml) was added to the reaction...
Embodiment 2
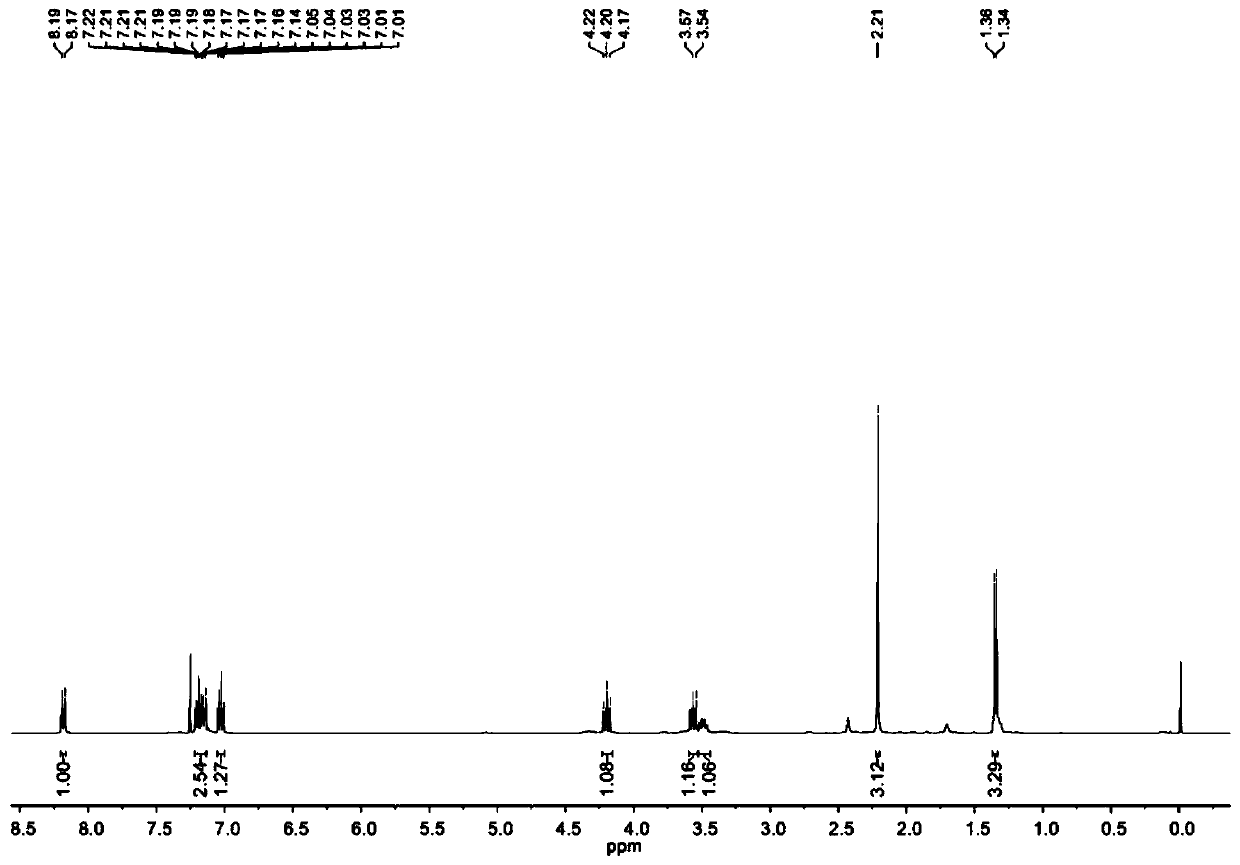
[0079] Embodiment 2: Starting from 2-iodoaniline, synthesis of N-acetyl-3-methylindoline
[0080]
[0081] 2-iodoaniline (2.19 g), potassium carbonate (2.76 g), dimethyl sulfoxide (DMSO, 50 ml) were added to a round-bottomed flask, and acetyl chloride (AcCl, 785 mg) was slowly added dropwise under ice-cooling. After the addition was complete, the ice bath was removed, and the resulting mixed system was raised to room temperature, and continued to stir for 12 hours; after that, allyl bromide (1.21 g) was added dropwise under the ice bath. Water (50 milliliters) quenches reaction, uses 50 milliliters of diethyl ether to extract 3 times, combines organic phase, removes solvent under reduced pressure on rotary evaporator and obtains N-acetyl-N-allyl-2-iodoaniline (2.77 grams ) crude product, this product can carry out next step reaction without further purification;
[0082] SmI 2 (0.1 mol / liter tetrahydrofuran solution, 120 milliliters) was added in the reaction tube, and tr...
Embodiment 3
[0086] Example 3: Synthesis of N-acetyl-3,5-dimethylindoline from 2-iodoaniline
[0087]
[0088]2-iodo-4-methylaniline (2.19 g), potassium carbonate (2.76 g), dimethyl sulfoxide (DMSO, 50 ml) were added to a round bottom flask, and acetyl chloride (AcCl, 785 mg), the dropwise addition was completed, and the ice bath was removed, and the resulting mixed system was raised to room temperature, and continued to stir for 12 hours; after that, continued dropwise addition of allyl bromide (1.21 g) under the ice bath, after the dropwise addition, room temperature was reacted for 16 hours Finally, add deionized water (50 ml) to quench the reaction, use 50 ml of ether to extract 3 times, combine the organic phases, and remove the solvent under reduced pressure on a rotary evaporator to obtain N-acetyl-N-allyl-2- Iodo-4-methylaniline (3.03 grams) crude product, this product can carry out next step reaction without further purification;
[0089] SmI 2 (0.1 mol / L tetrahydrofuran solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com