Method for preparing water body phosphate oxygen isotope sample through in-situ enrichment
An oxygen isotope and in-situ enrichment technology, which is applied in the preparation of test samples, sampling, and measuring devices, can solve the problems of consuming a lot of time and reagent materials, the workload of water collection, and the large error of results, etc., and achieve saving Effects of time and economic cost, reduction of water harvesting workload, and simplification of difficulty or steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1) Cover the Zr-Oxide gel membrane with a diameter of 10cm with a nylon mesh, and immerse it in the in-situ water body to be tested for phosphate enrichment until the required detection time period is reached;
[0043] 2) Take out the Zr-Oxide gel film and place it in 20 mL of 1mol / L sodium hydroxide solution for continuous shaking for 24 hours, and pass it through a 0.45 μm filter membrane to obtain a phosphorus-containing eluent;
[0044] 3) Adjust the pH value of the above-mentioned phosphorus-containing eluent to 5.8, then add cerium nitrate solution to adjust the pH value to 5.5, and get the cerium phosphate precipitate; the cerium nitrate solution is prepared from 0.5g cerium nitrate hexahydrate and 2mL water;
[0045] 4) Add 15 mL of 0.2 mol / L nitric acid to the above cerium phosphate precipitate, and then add 5 mL of Biorad AG 50W-X8 cation exchange resin after the cerium phosphate precipitate is completely dissolved and shake continuously for 12 hours to remove ...
Embodiment 2
[0049] In order to further eliminate Cl - Content, on the basis of Example 1, also can be in step 4) in, before adding the nitric acid of 15mL 0.2mol / L, wash the cerium phosphate precipitate three times continuously with the buffer solution that pH value is 5.6 earlier, described buffer solution consists of It is prepared by mixing 1L of pure water with 0.2mL of 99.8% glacial acetic acid and then adding 1mol / L of potassium acetate.
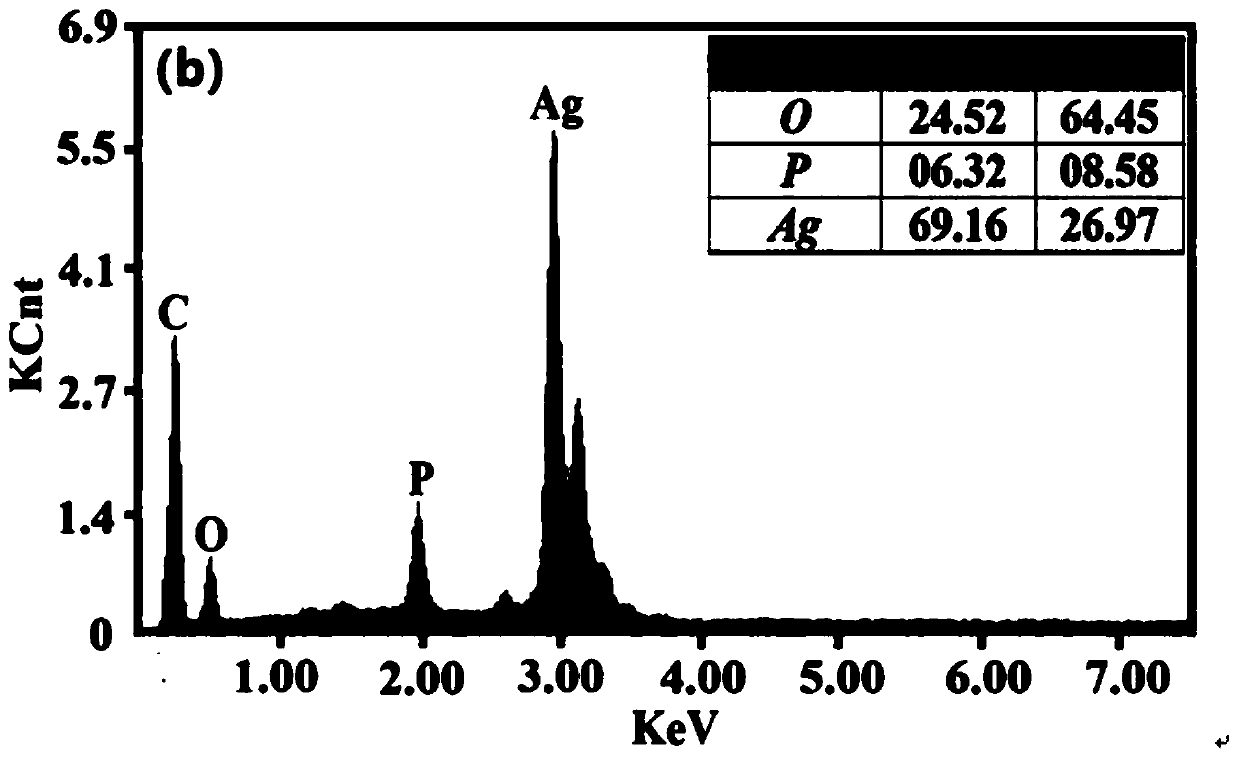
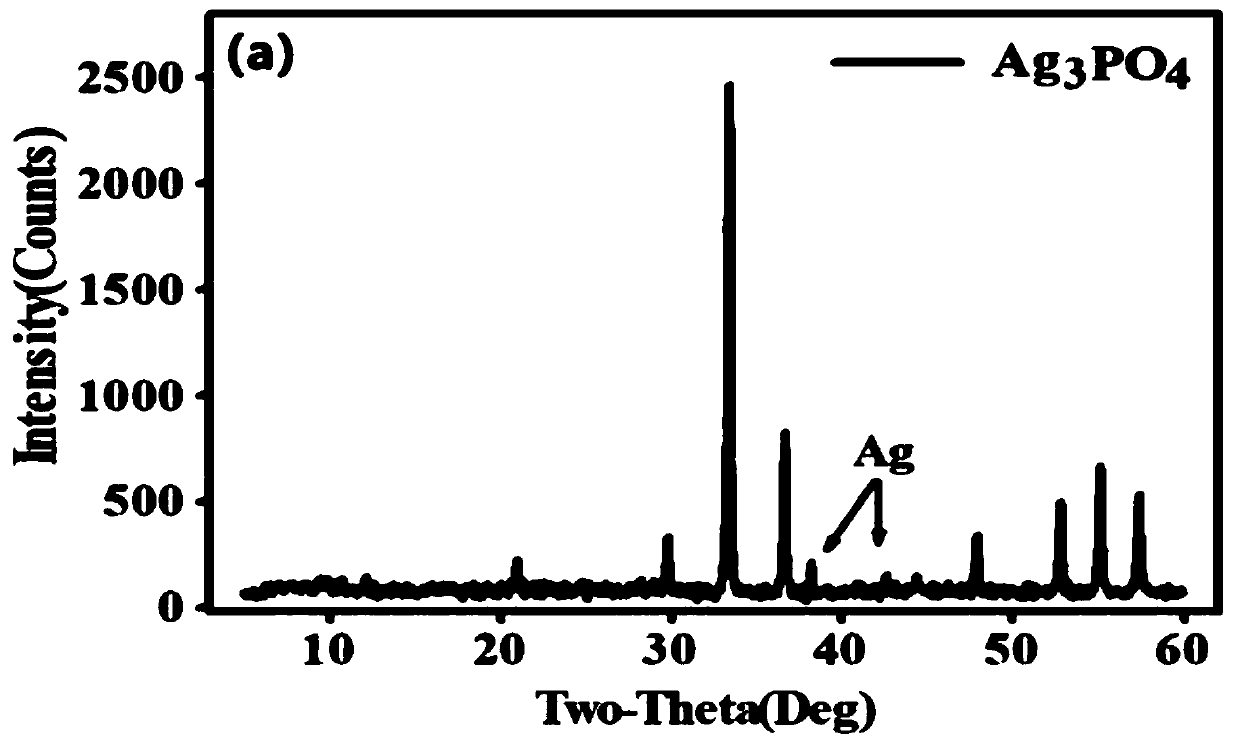
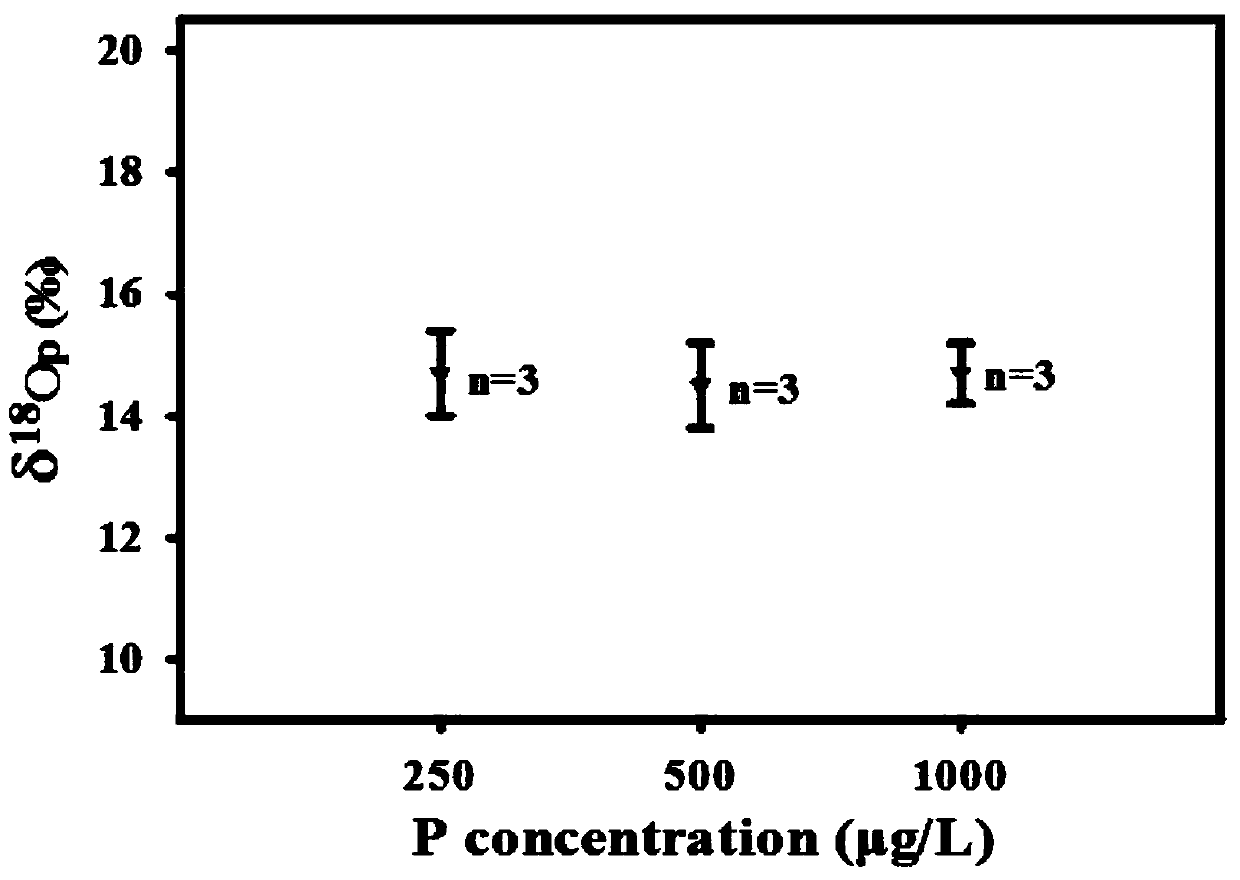
[0050] from Figure 1~3 It can be seen that the Ag prepared by the method of the present invention 3 PO 4 The sample has good purity and only contains a very small amount of Ag ((this is because Ag 3 PO 4 It has the characteristics of easily decomposing a small amount of nano-silver when exposed to light, but does not affect the δ 18 o P Composition test results). Water body δ at three phosphorus concentration levels 18 o P The composition information is relatively close, and they are all added from the same phosphorus source (KH 2 PO 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com