Platinum-based intermetallic nanocrystalline oxygen reduction catalyst and preparation method thereof
A base metal, nanocrystalline technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve problems such as unfavorable large-scale, industrialized production, complex catalyst preparation process, and easy dissolution of transition metals, etc. problems, to achieve the effect of easy mass production and application, easy mass production, and improved electrochemical active area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] An embodiment of the present invention provides a method for preparing a platinum-based intermetallic nanocrystalline oxygen reduction catalyst, such as figure 1 As shown, including the following steps:
[0031] S1 Dissolve the reducing agent in the organic amine solution to obtain a reducing agent solution with a concentration of 0.05 to 0.2 mol / L. Dissolve the platinum salt in the organic amine solution and stir at 80°C to 110°C for 10 to 30 minutes to obtain a concentration of 0.005~0.02mol / L platinum salt solution; add the reducing agent solution to the platinum salt solution to obtain a mixed solution, so that the molar ratio of platinum salt and reducing agent is 1:40~2:5 (generally, the more the reducing dose, the more The higher the nuclear rate, the smaller the final nanoparticle size). After 0.5 to 1 hour, the platinum salt and the reducing agent fully react to obtain platinum nanoparticles, which are then centrifuged and washed to separate platinum nanoparticles...
Embodiment 1
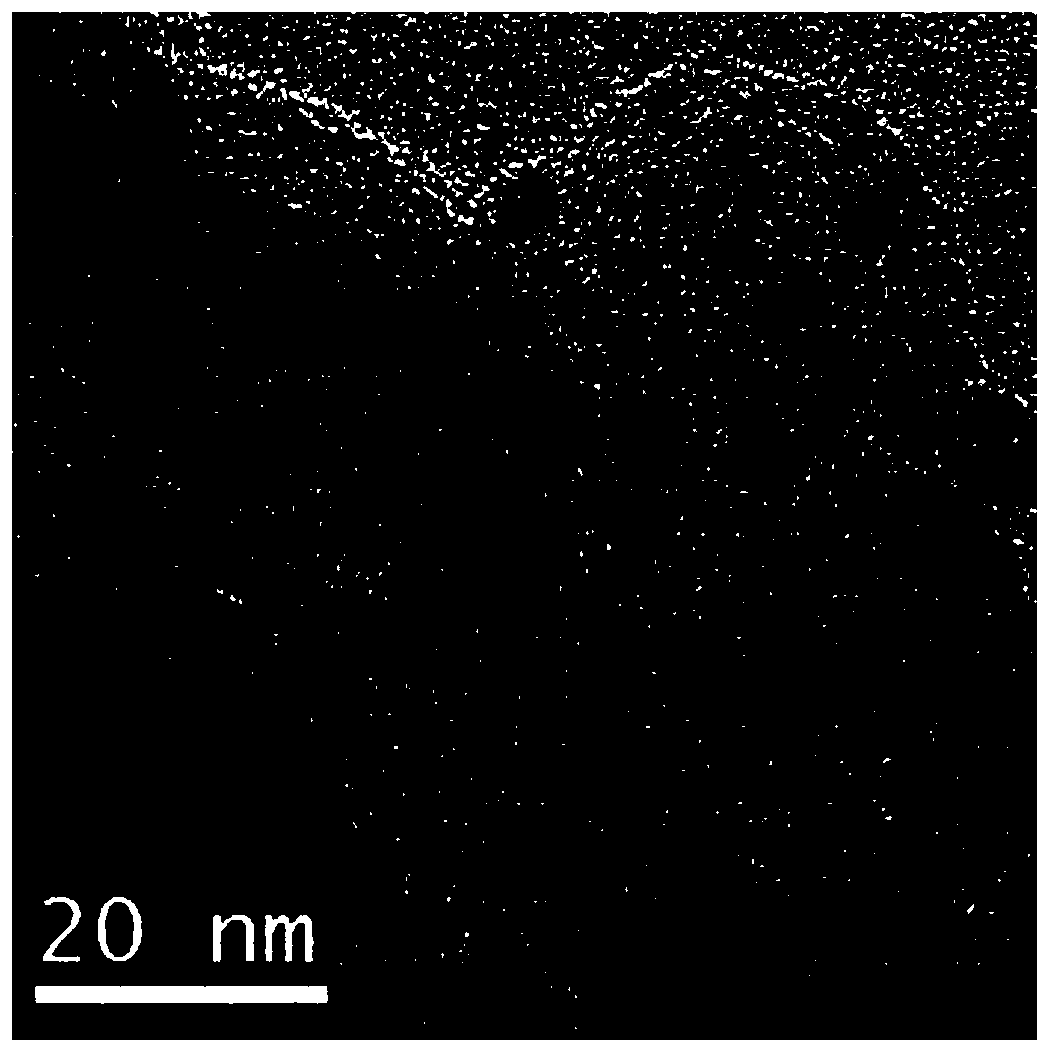
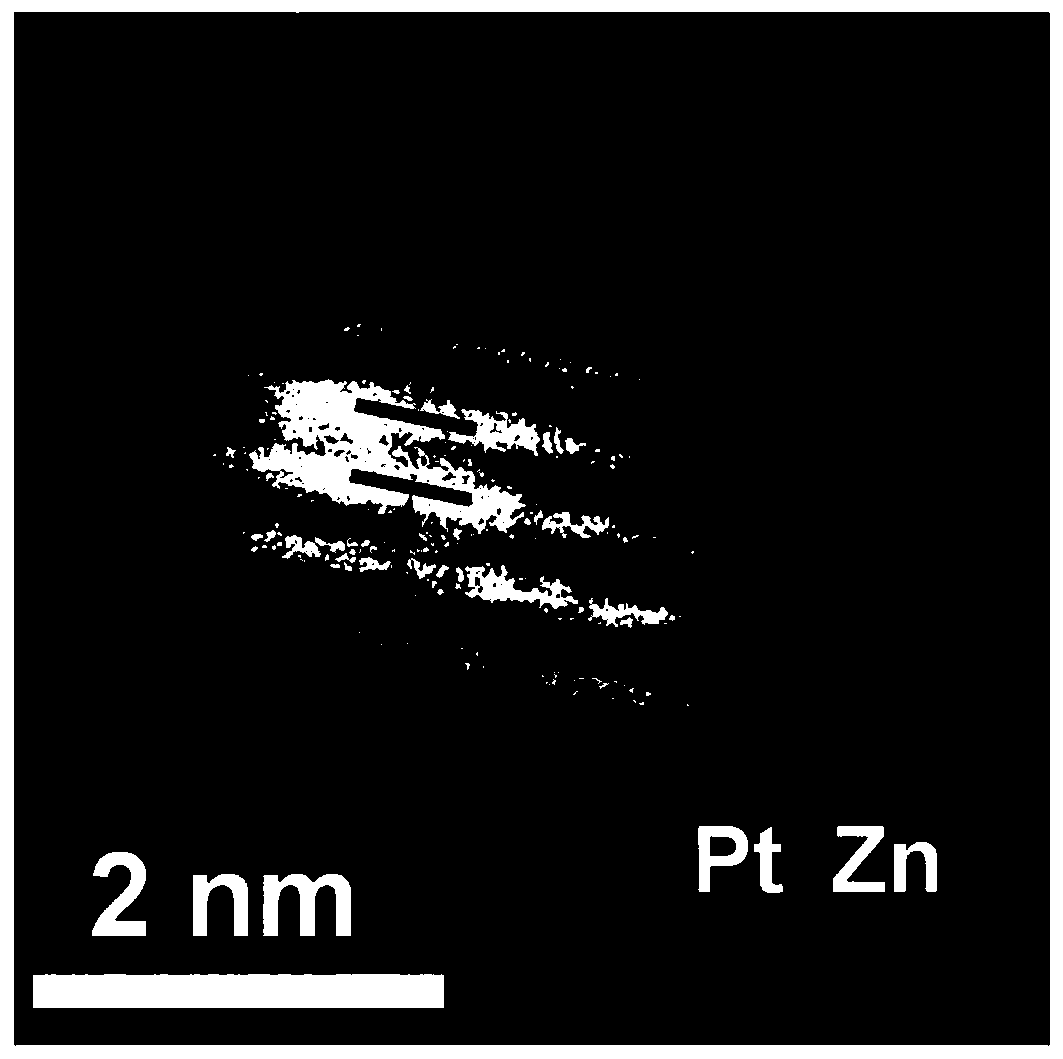
[0039] S1 Take platinum acetylacetonate and dissolve it in 5mL oleylamine solution to prepare a solution with a platinum precursor concentration of 0.01mol / L. After the solution is stirred at 100°C for 20 minutes, the concentration of 0.2mol / L borane-tert-butylamine The oleylamine solution was added to the above solution, the amount was 5mL, then the solution was heated to 120°C, the reaction was continued for 1 hour, and then centrifuged and washed to obtain ultrafine platinum nanoparticles;
[0040] S2 Disperse a certain amount of zinc acetate and the above synthesized ultrafine platinum nanoparticles in 5 mL of oleylamine solution to form a zinc salt precursor concentration of 0.2mol / L solution, and then the above solution is reacted at 270℃ for 1 hour , Centrifugal washing, the zinc oxide protective layer can be coated on platinum nanoparticles;
[0041] In S3, the platinum / zinc oxide composite obtained was annealed at 600°C for 2 hours in an argon-hydrogen atmosphere. After co...
Embodiment 2
[0043] S1 Take platinum acetylacetonate and dissolve it in 5 mL of oleylamine solution to prepare a solution with a platinum precursor concentration of 0.01 mol / L. After the solution is stirred at 100°C for 20 minutes, the concentration of 0.05 mol / L borane-tert-butylamine The oleylamine solution was added to the above solution, the amount was 5mL, then the solution was heated to 120°C, the reaction was continued for 1 hour, and then centrifuged and washed to obtain platinum nanoparticles;
[0044] S2 Take the platinum nanoparticles and zinc acetate prepared above and dissolve them in 5 mL of oleylamine solution to prepare a solution with a zinc salt concentration of 0.2 mol / L. After the solution is reacted at 270°C for 1 hour, it is centrifuged and washed. The particles are coated with a protective layer of zinc oxide;
[0045] In S3, the platinum / zinc oxide composite obtained was annealed at 700℃ for 2 hours under an argon hydrogen atmosphere. After cooling, pickling, and ultraso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com