Application of immobilized carboxyester hydrolase in side chain synthesis of cloxacillin, dicloxacillin, flucloxacillin and oxacillin
A technology for flucloxacillin, oxacillin, and carboxyl ester hydrolase, which is applied in the directions of immobilized enzymes, hydrolase, enzymes, etc., can solve the problems of high cost, low yield and high energy consumption, and achieves low cost and economical efficiency. Significant benefits and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The application of a kind of immobilized carboxyl ester hydrolase provided in the embodiment of the present invention in the synthesis of cloxacillin side chain, its chemical reaction formula is respectively:
[0019] .
Embodiment 2
[0021] The application of a kind of immobilized carboxyl ester hydrolase provided in the embodiment of the present invention in dicloxacillin side chain synthesis, its chemical reaction formula is respectively:
[0022] .
Embodiment 3
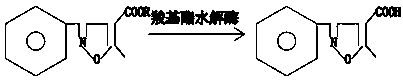
[0024] The application of a kind of immobilized carboxyl ester hydrolase in the synthesis of flucloxacillin side chain that the embodiment of the present invention provides, its chemical reaction formula is respectively:
[0025] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com