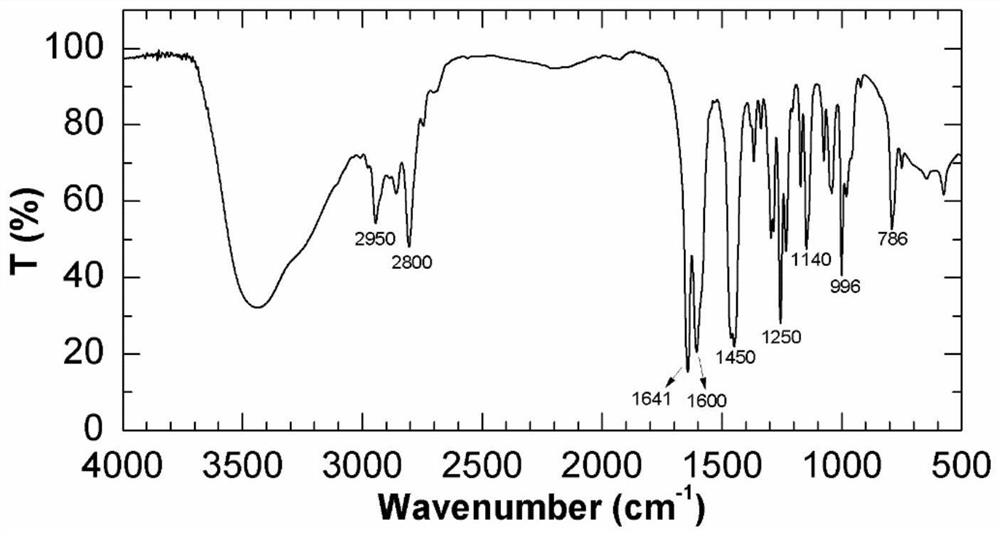
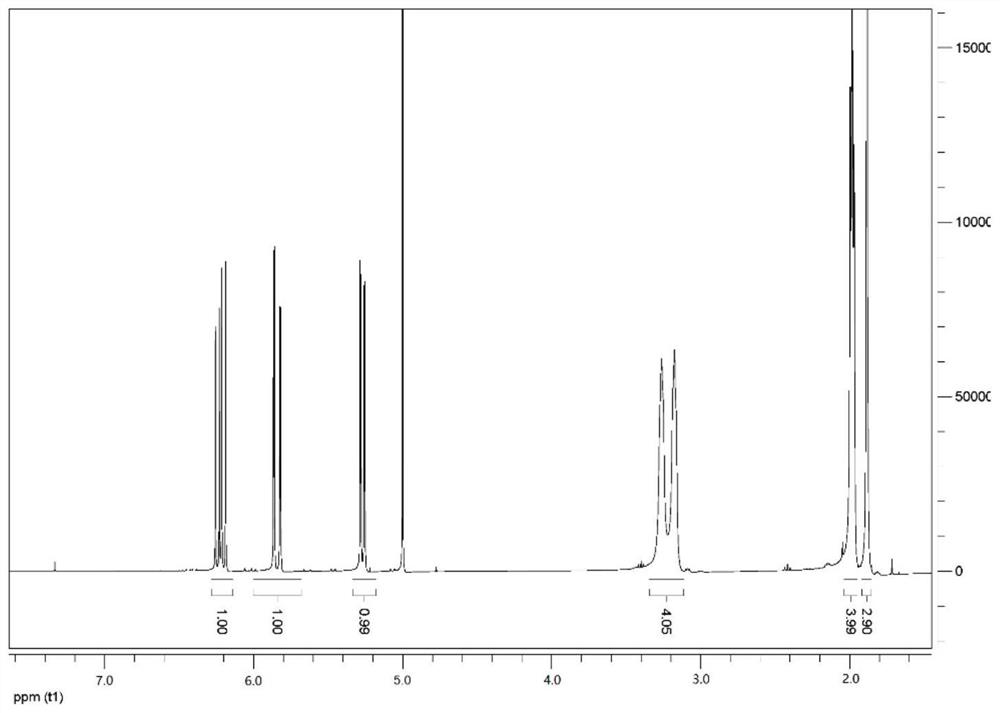
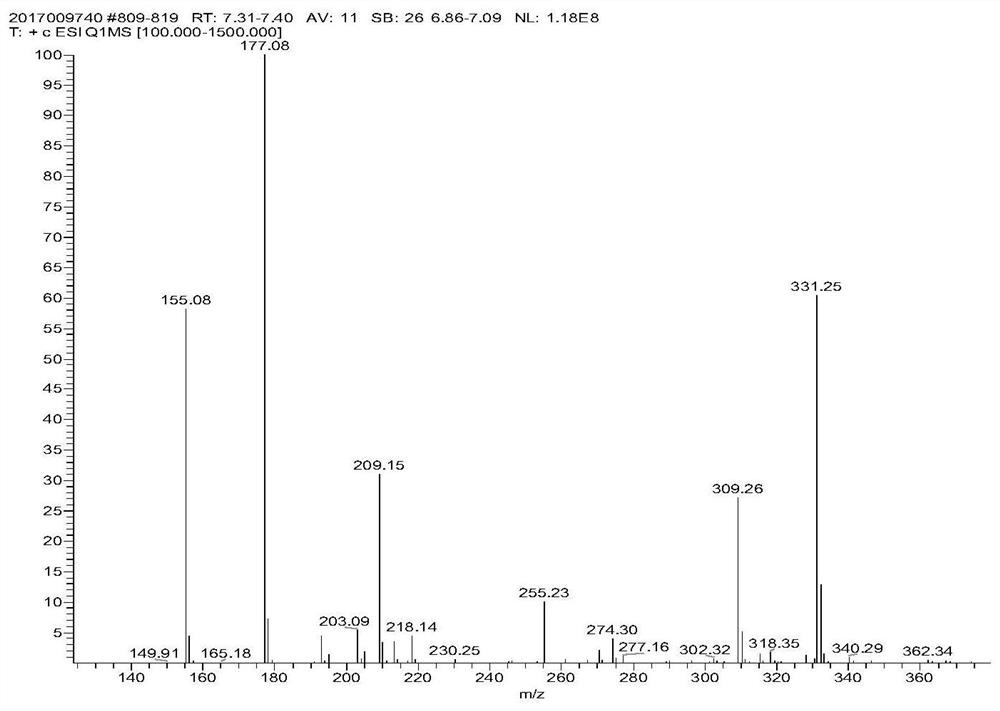
A kind of functional monomer for synthesizing polymer oil displacement agent and preparation method thereof
A technology for synthesizing polymers and functional monomers, applied in drilling compositions, chemical instruments and methods, organic chemistry, etc., can solve problems such as increased difficulty in process control, failure to successfully polymerize, and insufficient polymerization activity, and achieve improved Effects of yield and purity, stable chemical structure, and high polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 (large production reaction of 10L)
[0053] In this example, R 1 For -H, the corresponding acid chloride is acryloyl chloride; R 2 for -CH 3 , and the corresponding piperazine derivative is N-methylpiperazine. The specific implementation steps of this embodiment are as follows:
[0054] (1) Add 5 L of dichloromethane and 1.1 kg of acryloyl chloride (12 mol) into a 10 L reactor in sequence. At 5° C., 1 kg of N-methylpiperazine (10 mol) was slowly added using a constant pressure dropping funnel.
[0055] (2) After the dropwise addition is completed, slowly warm up to room temperature, and continue to react for 10h; during the dropwise addition, yellow flocculent insolubles will gradually be produced, which is due to the formation of hydrochloride by the reaction by-product HCl and the functional monomer. due to low solubility in dichloromethane.
[0056] (3) After the reaction was completed, 1 L of deionized water was added, followed by saturated NaOH s...
Embodiment 2
[0064] In this example, R 1 for -CH 3 , the corresponding acid chloride is methacryloyl chloride; R 2 for -CH 3 , and the corresponding piperazine derivative is N-methylpiperazine. The specific implementation steps of this embodiment are as follows:
[0065] (1) Add 250 mL of dichloromethane and 62.72 g of methacryloyl chloride (0.6 mol) into a 500 mL three-necked flask in sequence. At 0° C., 50 g of N-methylpiperazine (0.5 mol) was added dropwise with a constant pressure dropping funnel.
[0066] (2) After the dropwise addition was completed, the temperature was slowly raised to room temperature, and the reaction was continued for 6 h. During the dropwise addition, yellow flocculent insolubles will gradually be produced, which is caused by the low solubility in dichloromethane after the reaction by-product HCl forms hydrochloride with the functional monomer.
[0067] (3) After the reaction was completed, 50 mL of deionized water was added, followed by saturated NaOH sol...
Embodiment 3
[0070] In this example, R 1 For -H, the corresponding acid chloride is acryloyl chloride; R 2 for -CH 2 CH 3 , and the corresponding piperazine derivative is N-ethylpiperazine. The specific implementation steps of this embodiment are as follows:
[0071] (1) 250 mL of dichloromethane and 54.3 g of acryloyl chloride (0.6 mol) were sequentially added into a 500 mL three-necked flask. At 0°C, 57.1 g of N-ethylpiperazine (0.5 mol) was added dropwise with a constant pressure dropping funnel.
[0072] (2) After the dropwise addition was completed, the temperature was slowly raised to room temperature, and the reaction was continued for 1 h. During the dropwise addition, yellow flocculent insolubles will gradually be produced, which is caused by the low solubility in dichloromethane after the reaction by-product HCl forms hydrochloride with the functional monomer.
[0073] (3) After the reaction was completed, 50 mL of deionized water was added, followed by saturated NaOH solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com