Mn<4+>-doped hexafluoride red fluorescent powder and synthesis method thereof
A technology of red phosphor and synthesis method, applied in the field of hexafluoride-doped Mn4+ red phosphor and synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add hexafluorosilicic acid to 2.5mL hydrofluoric acid solution (40% wt), then add 0.03g potassium hexafluoromanganate and stir for 30 minutes, and finally add 1.86g tetramethylammonium fluoride and continue stirring for 8 hours; the obtained precipitate Wash each 3 times with glacial acetic acid and absolute ethanol, and finally place the product in a vacuum oven and dry it for 12 hours, and the orange-yellow powder obtained is the final product [(CH 3 ) 4 N] 2 Si 1-x f 6 :xMn 4+ .
[0025] attached figure 1 for [(CH 3 ) 4 N] 2 Si 1-x f 6 :xMn 4+ The XRD diffraction pattern of this sample shows that the powder diffraction peaks of this sample are a series of independent narrow peaks, indicating that the sample has good crystallinity.
[0026] attached figure 2Shown as [(CH 3 ) 4 N] 2 Si 1-x f 6 :xMn 4+ The scanning electron microscope picture of the sample particle size is 2-5 μm.
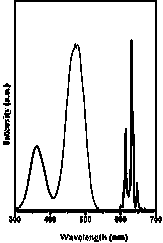
[0027] attached image 3 Shown are the room temperature excitation...
Embodiment 2
[0030] First add 0.13 g of germanium dioxide to 2.5 mL of hydrofluoric acid solution (40% wt), then add 0.03 g of potassium hexafluoromanganate and stir for 30 minutes, and finally add 1.86 g of tetramethylammonium fluoride and continue stirring for 8 hours; the resulting precipitate The product was washed 3 times with glacial acetic acid and absolute ethanol, and finally the product was placed in a vacuum drying oven for 12 hours, and the orange-yellow powder obtained was the final product [(CH 3 ) 4 N] 2 Ge 1-x f 6 :xMn 4+ .
[0031] attached Figure 5 for [(CH 3 ) 4 N] 2 Ge 1-x f 6 :xMn 4+ The XRD diffraction pattern of this sample shows that the powder diffraction peaks of this sample are a series of independent narrow peaks, indicating that the sample has good crystallinity.
[0032] attached Figure 6 Shown as [(CH 3 ) 4 N] 2 Ge 1-x f 6 :xMn 4+ The scanning electron microscope picture of the sample particle size is 5-10 μm.
[0033] attached Figure ...
Embodiment 3
[0036] First add 0.49mL hexafluorotitanic acid into 2.5mL hydrofluoric acid solution (40% wt), then add 0.03g potassium hexafluoromanganate and stir for 30 minutes, and finally add 0.93g tetramethylammonium fluoride and continue stirring for 8 hours; the obtained The precipitate was washed 3 times with glacial acetic acid and absolute ethanol, and finally the product was placed in a vacuum drying oven for 12 hours, and the orange-yellow powder obtained was the final product [(CH 3 ) 4 N] 2 Ti 1-x f 6 :xMn 4+ .
[0037] attached Figure 9 for [(CH 3 ) 4 N] 2 Ti 1-x f 6 :xMn 4+ The XRD diffraction pattern of this sample shows that the powder diffraction peaks of this sample are a series of independent narrow peaks, indicating that the sample has good crystallinity.
[0038] attached Figure 10 Shown as [(CH 3 ) 4 N] 2 Ti 1-x f 6 :xMn 4+ The scanning electron microscope picture of the sample particle size is 3-5 μm.
[0039] attached Figure 11 Shown are the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com