Nitro-substituted indole compound and preparation method thereof
A compound and indole technology, applied in the field of organic chemistry and medicinal chemistry, can solve the problems of low total yield and efficiency, difficult one-step synthesis, etc., achieve high product yield, easy post-processing, and avoid residue effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
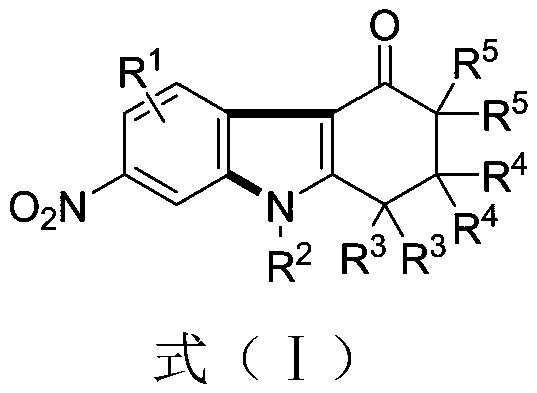
[0038] Example 1 Synthesis of 2,2-dimethyl-7-nitro-9-phenyl-1,2,3,9-tetrahydro-4H-carbazol-4-one
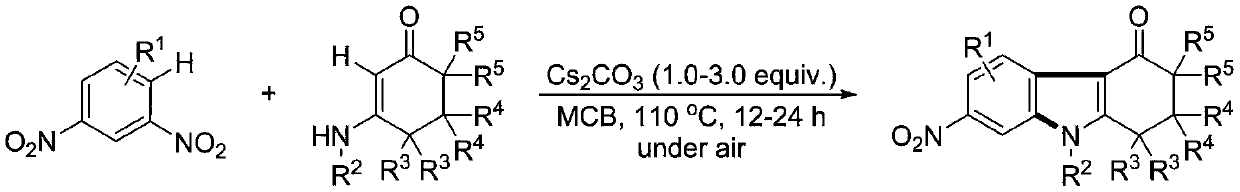
[0039] (1) Weigh 0.3mmol of 1,3-dinitrobenzene (0.0504 grams), 0.45mmol of 5,5-dimethyl-3-(phenylamino)cyclohexyl-2-enyl-1-ketone (0.0969 grams), 0.75 mmol of cesium carbonate (0.2444 grams), in a 10 mL test tube reaction tube, add 2 mL of m-dichlorobenzene (MCB) as a solvent, seal and seal, and stir and react at 110 ° C for 12 hours;
[0040] (2) After the reaction finishes, the reaction solution is successively dried by water, ethyl acetate, anhydrous sodium sulfate and separated by column chromatography (column chromatography separation condition: the stationary phase is 300~400 mesh silica gel powder, and the mobile phase is ethyl acetate Ester (A) and petroleum ether (B), the mobile phase change program (A:B) is 1:6→1:3, and 0.0622 g of the reaction product is obtained.
[0041] The above-mentioned reaction product is characterized, and the result is:
[0042] Colorless li...
Embodiment 2
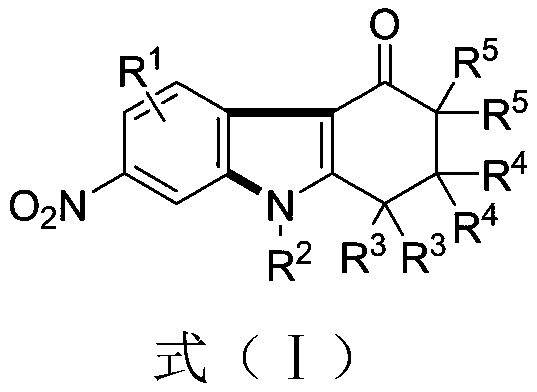
[0044] Example 2 Synthesis of 2,2-dimethyl-7-nitro-9-(4-methylphenyl)-1,2,3,9-tetrahydro-4H-carbazol-4-one
[0045] (1) Weigh 0.3mmol of 1,3-dinitrobenzene (0.0504 grams), 0.45mmol of 5,5-dimethyl-3-(4-methylphenylamino)cyclohexyl-2-enyl -1-ketone (0.1012 grams), 0.75 mmol of cesium carbonate (0.2444 grams), in a 10 mL test tube reaction tube, add 2 mL of m-dichlorobenzene (MCB) as a solvent, seal and seal, and stir at 110 ° C for 16 hours;
[0046] (2) After the reaction is over, the reaction solution is successively dried by water, ethyl acetate, anhydrous sodium sulfate and separated by column chromatography (column chromatography separation condition: the stationary phase is 300~400 mesh silica gel powder, and the mobile phase is ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B) is 1:6→1:3, and 0.0710 g of the reaction product is obtained.
[0047] The above-mentioned reaction product is characterized, and the result is:
[0048] Colorless ...
Embodiment 3
[0050] Example 3 Synthesis of 2,2-dimethyl-7-nitro-9-(4-methoxyphenyl)-1,2,3,9-tetrahydro-4H-carbazol-4-one
[0051] (1) Weigh 0.3mmol of 1,3-dinitrobenzene (0.0504 grams), 0.45mmol of 5,5-dimethyl-3-(4-methoxyphenylamino)cyclohexyl-2-ene Base-1-ketone (0.1104 g), 0.75 mmol of cesium carbonate (0.2444 g), in a 10 mL test tube reaction tube, add 2 mL of m-dichlorobenzene (MCB) as a solvent, seal it tightly, and stir at 110 ° C for 17 hours ;
[0052] (2) After the reaction is over, the reaction solution is successively dried by water, ethyl acetate, anhydrous sodium sulfate and separated by column chromatography (column chromatography separation condition: the stationary phase is 300~400 mesh silica gel powder, and the mobile phase is ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B) is 1:6→1:3, and 0.885 g of the reaction product is obtained.
[0053] The above-mentioned reaction product is characterized, and the result is:
[0054] colorless ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com