Heterocyclic modified two-photon polymerization initiator based on phenothiazine or carbazole and preparation method thereof
A technology of two-photon polymerization and phenothiazine, which is applied in the direction of organic chemistry, can solve the problems of insufficient additive manufacturing and low efficiency of two-photon polymerization initiators, and achieve the goal of short time required, good stability and high product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
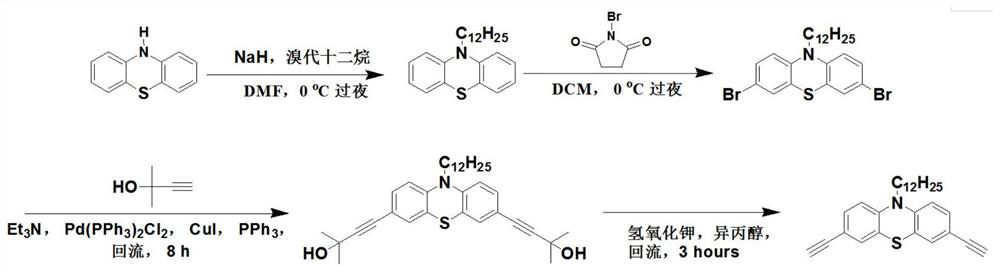
[0047] Step 1. Slowly add NaH (stored in 60% mineral oil, 3.00 g, 75 mmol) into an 80 mL DMF solution flask containing phenothiazine (10 g, 50 mmol), the flask is placed in a water bath containing ice-water mixture, and Stir, after 15 minutes, slowly add 1-bromododecane (14.4mL, 60mmol), stir the resulting solution overnight, then slowly add deionized water, after stirring for half an hour, extract with ethyl acetate, and then use column chromatography Purified by petroleum ether: ethyl acetate = 40:1 (v:v) eluent to obtain 10-dodecyl-10H-phenothiazine as light yellow oil 14.33g, yield 78% , with a purity of 96%;
[0048] Step 2. Slowly add NBS (8.9 g, 50 mmol) to a flask containing 10-dodecyl-10H-phenothiazine (7.35 g, 20 mmol) and 80 mL of DCM cooled to 0° C., and stir the resulting suspension overnight, Then, deionized water was slowly added and stirred for 30 minutes, then, saturated brine was added and extracted to obtain an organic phase, which was then dried with magne...
Embodiment 2
[0054]Step 1, NaH (stored in 60% mineral oil, 3.00g, 75mmol) was slowly added to the 80mL DMF solution flask containing phenothiazine (10g, 50mmol) cooled to 0°C, and stirred, after 15 minutes, 1-Bromododecane (14.4 mL, 60 mmol) was slowly added, and the resulting solution was stirred for 12 hours, then deionized water was slowly added, after stirring for half an hour, extracted with ethyl acetate, and then purified by column chromatography, using petroleum Ether: ethyl acetate = 40:1 (v:v) eluent to obtain 10-dodecyl-10H-phenothiazine as light yellow oil 14.15g with a yield of 77% and a purity of 95%;
[0055] Step 2. Slowly add NBS (8.9 g, 50 mmol) into a flask containing 10-dodecyl-10H-phenothiazine (7.35 g, 20 mmol) and 80 mL of DCM cooled to 0°C. The resulting suspension was stirred overnight, then deionized water was slowly added and stirred for 20 minutes, then saturated brine was added and extracted to obtain an organic phase, which was then dried over magnesium sulfat...
Embodiment 3
[0060] Step 1. Slowly add NaH (stored in 60% mineral oil, 4.00 g, 100mmol), after 15 minutes, 1-bromododecane (18.0mL, 75mmol) was slowly added, the resulting suspension was stirred overnight and deionized water was slowly added, filtered and washed with petroleum ether to obtain 3,6-di Bromo-9-dodecyl-carbazole, gray solid 21.61g, yield 88%, purity 96%;
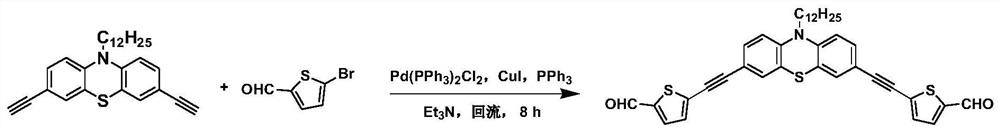
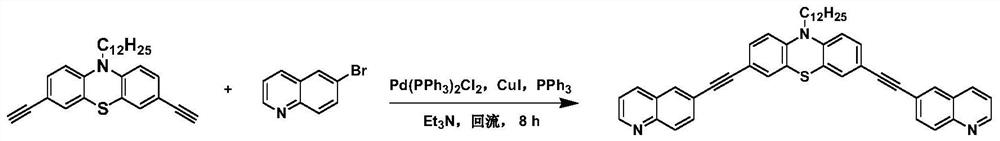
[0061] Step 2, add 3,6-dibromo-9-dodecyl-carbazole (4.93g, 10mmol), CuI (190mg, 1mmol), PdCl to the 250mL three-necked flask 2 (PPh 3 ) 2 (350mg, 0.5mmol), PPh 3 (262mg, 1mmol), 1,1-dimethyl-2-butyn-1-ol (2.91mL, 30mmol) and triethylamine (80mL), under the protection of Ar, the resulting mixture was refluxed at 90°C for 8 hours , after cooling, the solvent was removed, the residue was poured into saturated brine and extracted with ethyl acetate (5×100 mL), the organic layers were combined and washed with MgSO 4 Dry, concentrate, and use petroleum ether:ethyl acetate=10:1 (v:v) as eluent, purify by column chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com