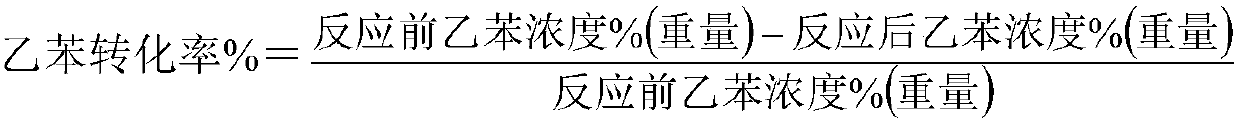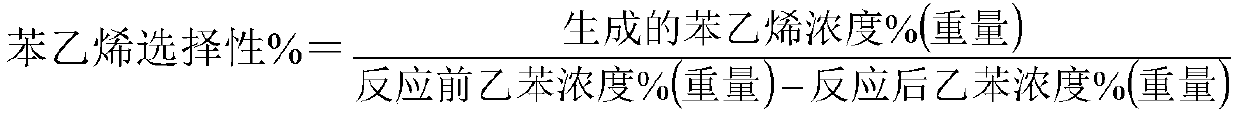Dehydrogenation catalyst for preparing styrene, preparation method and application thereof
A dehydrogenation catalyst and catalyst technology, applied in the direction of catalysts, carbon compound catalysts, chemical instruments and methods, etc., can solve the problems of high by-product toluene and low selectivity of low-potassium catalysts, and achieve less by-product toluene and selectivity for styrene sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] would be equivalent to 56.02 parts Fe 2 o 3 iron oxide red, equivalent to 15.69 parts of Fe 2 o 3 Iron oxide yellow, equivalent to 9.35 parts K 2 Potassium carbonate of O, equivalent to 8.7 parts CeO 2 of cerium oxalate, equivalent to 2.76 parts of WO 3 ammonium tungstate, calcium hydroxide equivalent to 3.3 parts of CaO, 2.86 parts of Sc 2 o 3 , 1.32 parts of HfO 2 Stir in a kneader with 5.2 parts of graphite for 2 hours, add deionized water accounting for 22.3% of the total weight of the catalyst raw material, stir for 0.8 hours, take out the extruded strips, extrude them into particles with a diameter of 3 mm and a length of 6 mm, and put them into an oven. ℃ for 2.5 hours, 130 ℃ for 3.0 hours, then placed in a muffle furnace, calcined at 650 ℃ for 3 hours, and 960 ℃ for 3 hours to obtain the finished catalyst. The composition of the catalyst is listed in Table 1.
[0040] 100 milliliters of catalysts are loaded into the reactor, at normal pressure, liquid sp...
Embodiment 2
[0048] Except for ZrO 2 replace HfO 2 Outside, catalyst preparation method and catalyst evaluation condition are with embodiment 1, specifically:
[0049] would be equivalent to 56.02 parts Fe 2 o 3 iron oxide red, equivalent to 15.69 parts of Fe 2 o 3 Iron oxide yellow, equivalent to 9.35 parts K 2 Potassium carbonate of O, equivalent to 8.7 parts CeO 2 of cerium oxalate, equivalent to 2.76 parts of WO 3 ammonium tungstate, calcium hydroxide equivalent to 3.3 parts of CaO, 2.86 parts of Sc 2 o 3 , 1.32 parts of ZrO 2 Stir in a kneader with 5.2 parts of graphite for 2 hours, add deionized water accounting for 22.3% of the total weight of the catalyst raw material, stir for 0.8 hours, take out the extruded strips, extrude them into particles with a diameter of 3 mm and a length of 6 mm, and put them into an oven. ℃ for 2.5 hours, 130 ℃ for 3.0 hours, then placed in a muffle furnace, calcined at 650 ℃ for 3 hours, and 960 ℃ for 3 hours to obtain the finished catalyst. ...
Embodiment 3
[0051] Except for TiO 2 replace HfO 2 Outside, catalyst preparation method and catalyst evaluation condition are with embodiment 1, specifically:
[0052] would be equivalent to 56.02 parts Fe 2 o 3 iron oxide red, equivalent to 15.69 parts of Fe 2 o 3 Iron oxide yellow, equivalent to 9.35 parts K 2 Potassium carbonate of O, equivalent to 8.7 parts of cerium oxalate, equivalent to 2.76 parts of WO 3 ammonium tungstate, calcium hydroxide equivalent to 3.3 parts of CaO, 2.86 parts of Sc 2 o 3 , 1.32 parts of TiO 2 Stir in a kneader with 5.2 parts of graphite for 2 hours, add deionized water accounting for 22.3% of the total weight of the catalyst raw material, stir for 0.8 hours, take out the extruded strips, extrude them into particles with a diameter of 3 mm and a length of 6 mm, and put them into an oven. ℃ for 2.5 hours, 130 ℃ for 3.0 hours, then placed in a muffle furnace, calcined at 650 ℃ for 3 hours, and 960 ℃ for 3 hours to obtain the finished catalyst. The com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com