A kind of zearalenone degrading enzyme mutant with improved thermostability and application thereof
A technology of zearalenone and thermal stability, applied in the field of bioengineering, can solve problems such as low removal efficiency, unfavorable environmental protection, animal and plant health, and secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 Preparation of Zearalenone Degrading Enzyme Mutant
[0029] Select zearalenone-degrading enzymes from different microbial sources, analyze the differences in their thermal stability, and compare their respective amino acid sequences; at the same time, based on the crystal structure that has been resolved, analyze the The residues are exactly in the "cap" region and the core catalytic region in the ligase structure. For this reason, different ways of site-directed mutagenesis were chosen for the 134-position and 136-position residues.
[0030] Construction of pET-22b(+)-H134L / S136L mutant plasmid:
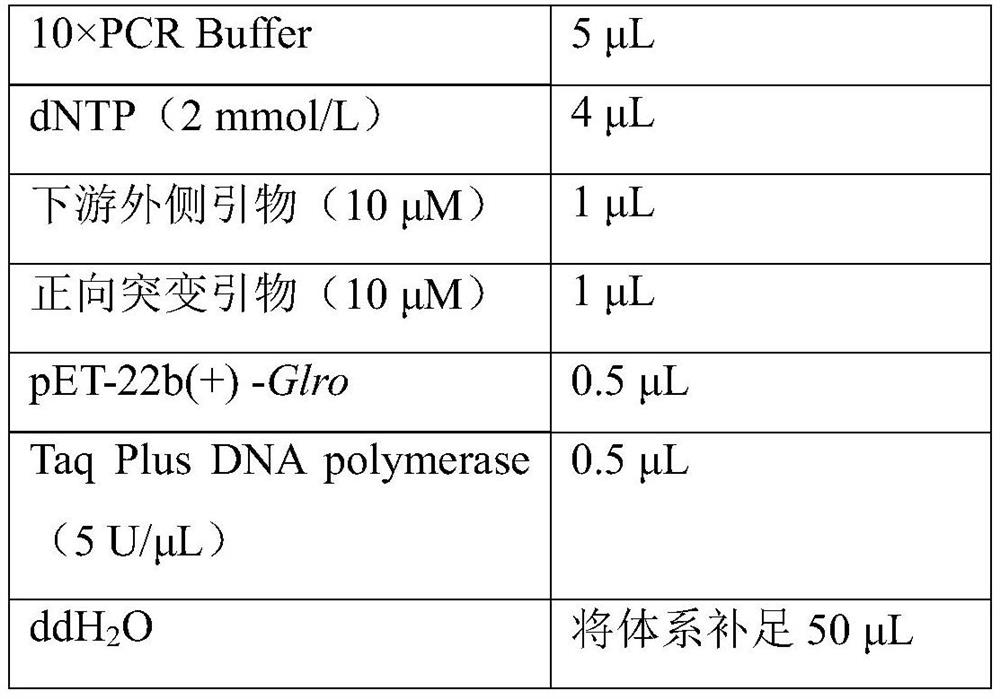
[0031] According to the gene encoding zearalenone degrading enzyme derived from Gliocladium roseum MA918 (accession number: KR363960.1), the gene Glro encoding the wild enzyme of zearalenone degrading enzyme was synthesized and linked to the pET-22b (+) Recombinant plasmid pET-22b(+)-Glro was obtained between restriction site Nde I and Xho I; using pET-22b(+)-G...
Embodiment 2
[0050] Example 2 Expression and purification of zearalenone degrading enzyme mutant
[0051] Transform the mutant plasmid pET-22b(+)-H134L / S136L into Escherichia coli BL21(DE3) cells, pick the positive transformants and culture them overnight in LB medium at 37°C and 200rpm in shake flasks, and then insert them into LB medium at 37°C Cultivate for 3-4 hours until the OD value is 0.6-0.8, cool down to 28°C, and add IPTG at a final concentration of 0.6mM to induce for 6 hours.
[0052] The fermentation broth was centrifuged at 4°C and 10,000 rpm for 20 min to obtain bacterial cells. Add 20mL buffer (50mM Tris, 200mM NaCl, HCl to adjust the pH to 8.5) to fully resuspend the bacteria, then place the centrifuge tube in an ice bath and put it into an ultrasonic cell disruptor. The conditions for ultrasonic disruption are: working time 1s , stop time 2s, a total of 18min. The obtained crushed solution was subjected to low-temperature high-speed centrifugation at 4°C and 10,000 rpm ...
Embodiment 3
[0054] Example 3 Determination of Thermal Stability of Zearalenone Degrading Enzyme Mutant H134L / S136L
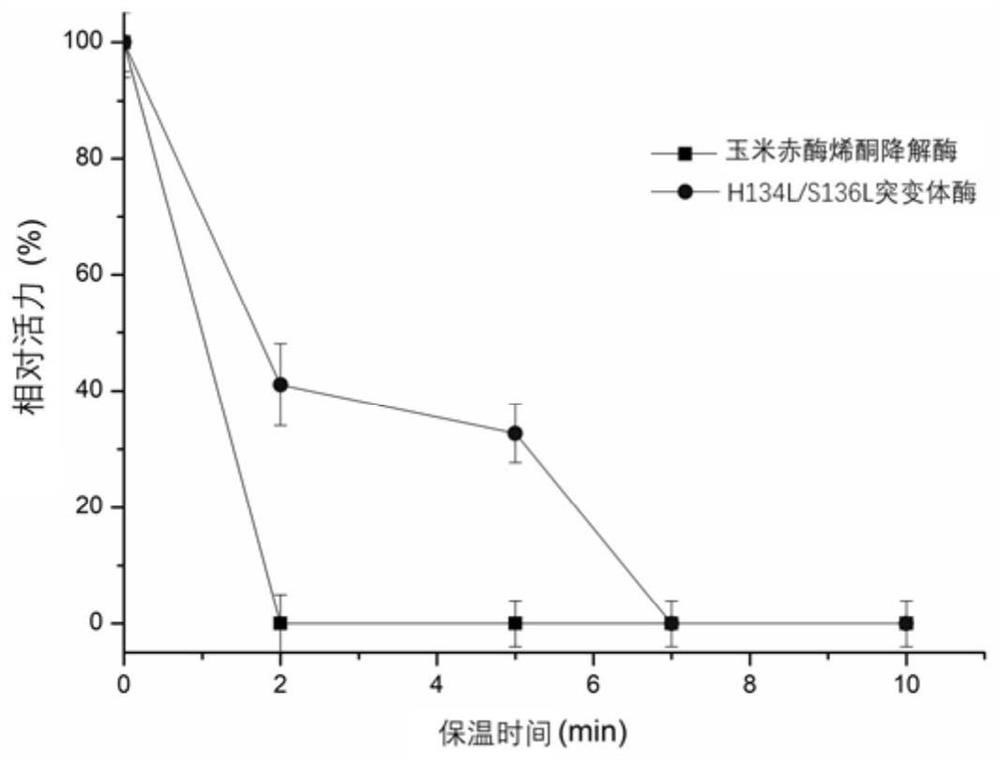
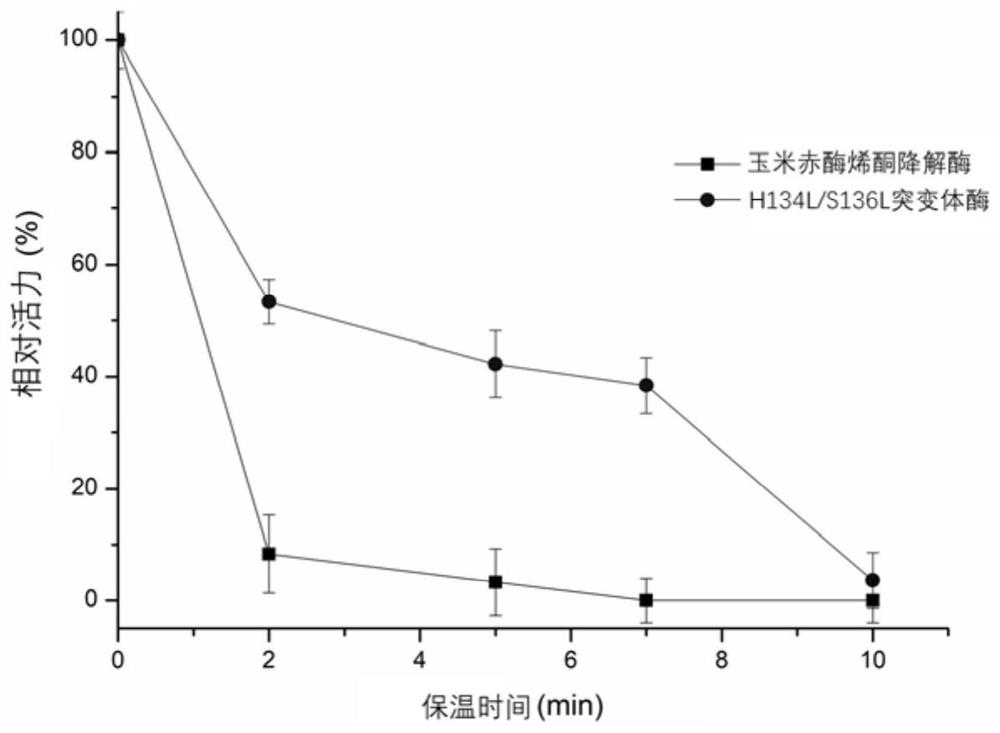
[0055] The wild enzyme and the mutant H134L / S136L were incubated at 53°C and 58°C for a period of time to measure the remaining enzyme activity. The initial enzyme activity at 0 time of incubation at 53°C and 58°C was 100%, and the specific enzyme activity was 118U / mg and 47U / mg. Such as figure 1 and figure 2 As shown, compared with the wild enzyme, the optimal catalytic conditions of mutant H134L / S136L did not change, but the residual enzyme activity of mutant H134L / S136L increased by 45% after incubation at 53°C for 2 minutes; the residual enzyme activity after incubation for 5 minutes Increased by 38%; after incubation for 7 minutes, the residual enzyme activity increased by 38%. After incubation at 58°C for 2 minutes, the residual enzyme activity increased by 41%, and after incubation for 5 minutes, the residual enzyme activity increased by 32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com