Application of fecal flora in preparing micro-ecological preparation for treating chronic hepatitis B
A technology of chronic hepatitis B and fecal flora, applied in the fields of application, antiviral agents, allergic diseases, etc., to achieve the effect of promoting the reduction of HBsAg titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the flora capsule can be: pack the flora liquid obtained in step 1) with a capsule material, pack it and store it at -80°C to form a flora capsule; the capsule material It is the way of adopting double-layer suit.
[0043] The feces flora can be used in the preparation of medicines for treating HBeAg-negative chronic hepatitis B. It can be used in combination with other antiviral drugs such as entecavir, as long as they do not produce other adverse effects, such as allergic reactions.
[0044] The feces flora can be used in the preparation of medicines for alleviating the disease response of patients with chronic hepatitis B. The remission is not limited to changes in indicators such as ALT, AST, and HBsAg.
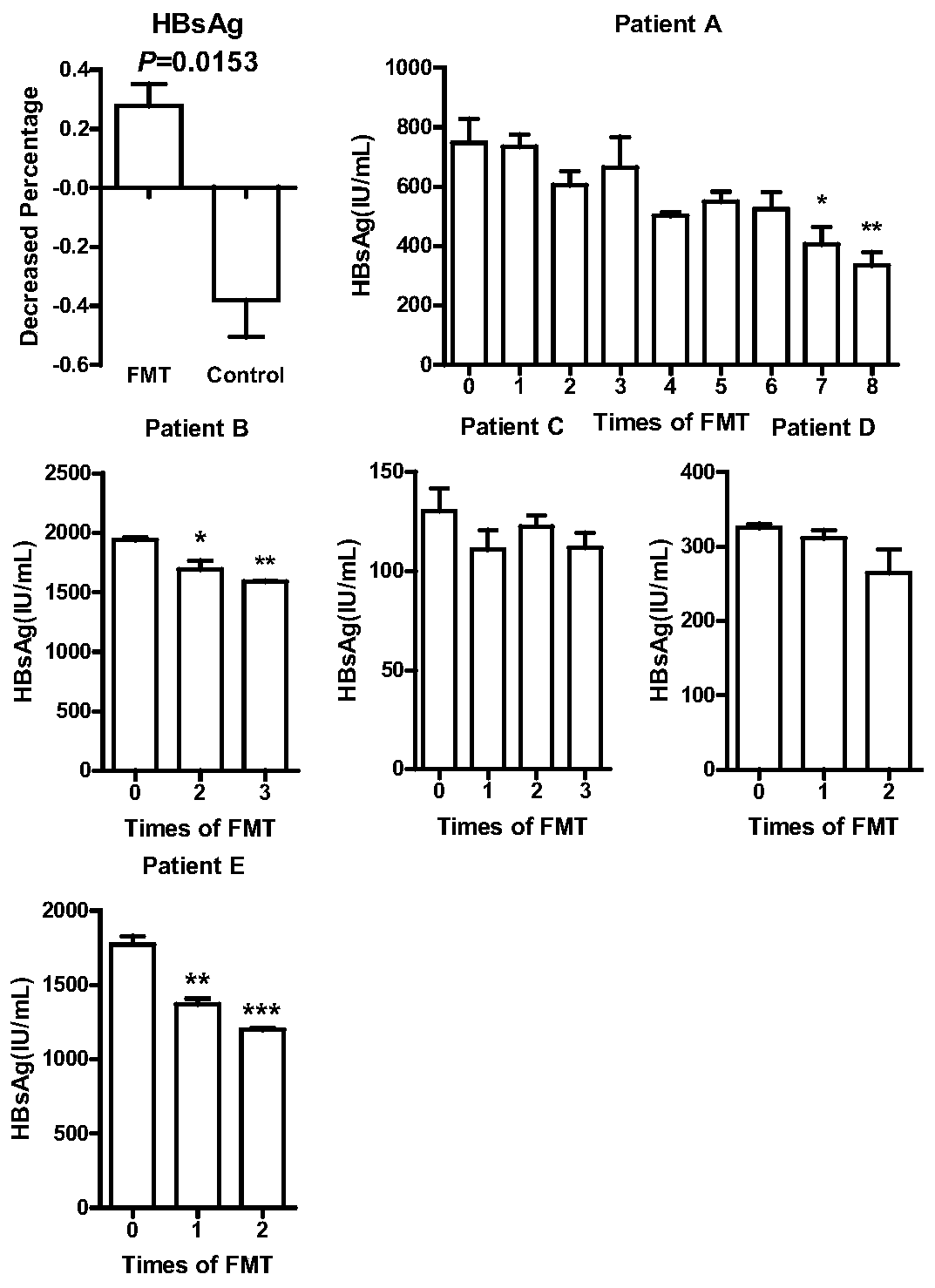
[0045] The feces flora can be used in the preparation of medicines for reducing HBsAg content in patients with chronic hepatitis B.
[0046] The feces flora can be used in the preparation of medicines for regulating the immune function ...
Embodiment 1
[0052] Example 1: Isolation and extraction of fecal flora from healthy donors
[0053] Strictly follow the inclusion and exclusion criteria of healthy donors to select suitable donors. Dissolve 100-200 g of the collected donor feces in 750-1000 mL of 0.9% NaCl solution, and remove the residue through the feces analysis pretreatment instrument TG-01 produced by Xiamen Chengge Biotechnology Co., Ltd. and supporting consumables to obtain fecal bacteria 30 ~40g, and prepared into oral flora capsules.
Embodiment 2
[0054] Embodiment 2: Patient's grouping and clinical application
[0055] 1. Inclusion criteria of patients
[0056] 1. Age 18-65 years old;
[0057] 2. No drinking history or equivalent alcohol consumption <140g per week for men and <70g per week for women;
[0058] 3. Meet the diagnostic criteria for HBeAg-negative chronic hepatitis B in the guidelines for the prevention and treatment of chronic hepatitis B in my country;
[0059] 4. Conform to the indications for antiviral drug treatment in the guidelines for the prevention and treatment of chronic hepatitis B in my country;
[0060] 5. HBsAg positive after standard antiviral drug treatment for more than 1 year;
[0061] 6. Serum transaminase and glutamyl aminotransferase are within the upper limit of the normal value, or the increase is less than 10 times the normal value;
[0062] 7. Those who voluntarily participate in this research and sign the informed consent form.
[0063] Note: Patients who meet the above 7 ite...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com