Zinc sulfate impurity removal method
A technology of zinc sulfate and zinc sulfate solution, applied in the direction of zinc sulfate, etc., can solve the problems of excessive zinc powder and high cost, and achieve the effect of rapid response, low cost and obvious impurity removal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
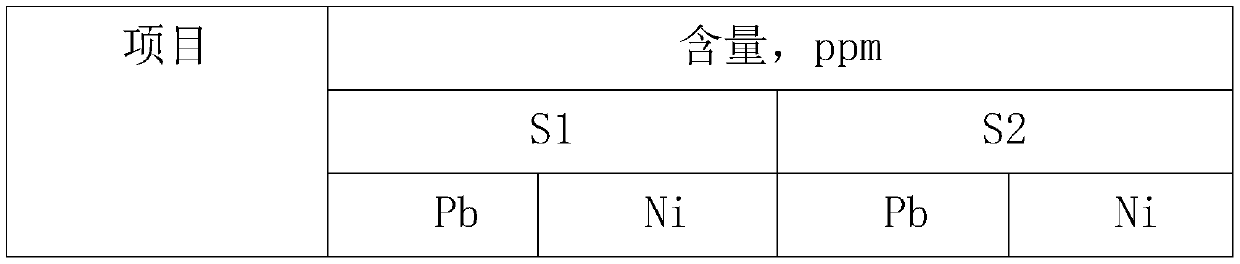
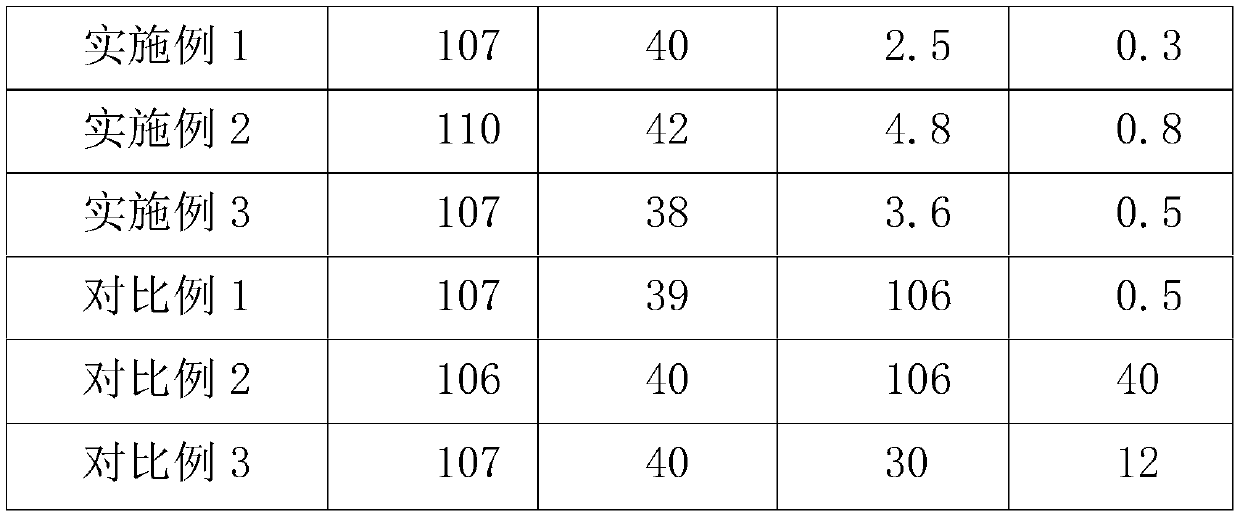
[0025] S1. The zinc source reacts with sulfuric acid to form a crude zinc sulfate solution, which is filtered and preliminarily removed to detect the content of lead and nickel;
[0026] S2. The temperature of the zinc sulfate solution was raised to 60° C., and a mixed solution of sodium thiocarbamate and thiourea with a nickel content of 15 times was added by weight ratio, stirred for 1.5 hours, and filtered to obtain a refined zinc sulfate solution.
[0027] Wherein the mixed solution of sodium thiourea and sodium thiourea is, by weight, sodium thiourea: the ratio of water is 1: 1: 2.
Embodiment 2
[0029] S1. The zinc source reacts with sulfuric acid to form a crude zinc sulfate solution, which is filtered and preliminarily removed to detect the content of lead and nickel;
[0030] S2. The temperature of the zinc sulfate solution was raised to 65° C., and a mixed solution of sodium thiocarbamate and thiourea with a nickel content of 15 times was added by weight ratio, stirred for 1 hour, and filtered to obtain a refined zinc sulfate solution.
[0031] Wherein the mixed solution of sodium thiourea and sodium thiourea is, by weight, sodium thiourea: the ratio of water is 1: 1: 4.
Embodiment 3
[0033] S1. The zinc source reacts with sulfuric acid to form a crude zinc sulfate solution, which is filtered and preliminarily removed to detect the content of lead and nickel;
[0034] S2. Heat the zinc sulfate solution to 65° C., add a mixed solution of sodium thiocarbamate and thiourea with a nickel content of 12 times by weight, stir for 1.5 hours, and filter to obtain a refined zinc sulfate solution.
[0035] Wherein the mixed solution of sodium thiourea and sodium thiourea is, by weight, sodium thiourea: the ratio of water is 1: 1: 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com