Application of iguratimod in preparation of medicine for treating systemic sclerosis
A systemic sclerosis and drug technology, applied in the field of biomedicine, can solve the problems of large side effects and poor scleroderma effect, and achieve the effect of obvious technical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
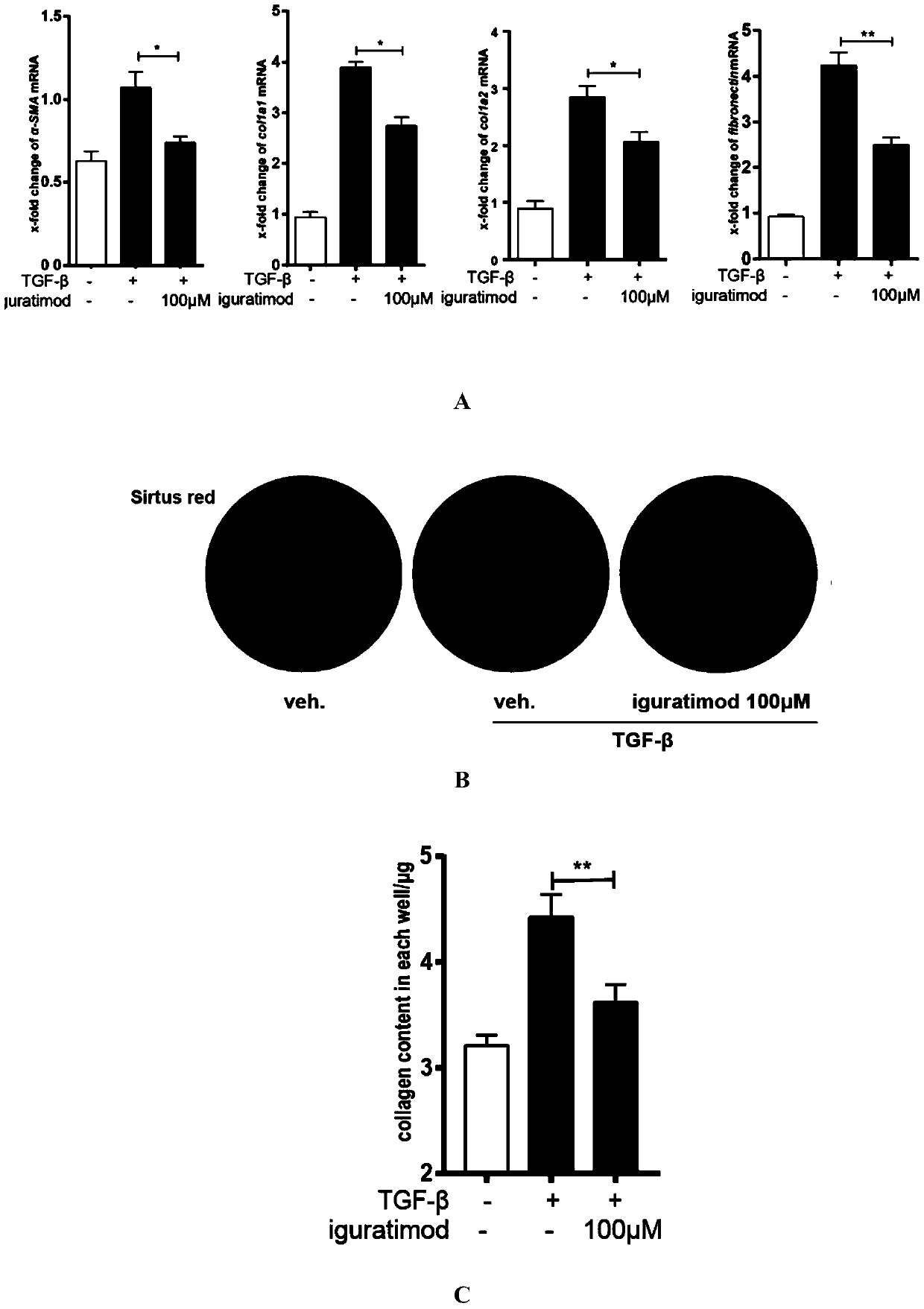
[0024] The present invention successfully extracts primary skin fibroblasts from normal adult skin tissue (20-year-old male, foreskin), cultures them, and transfers them to the third generation for experimentation. The present invention confirms that stimulating fibroblasts with TGF-β can promote the high expression of the transcription factor Egr1 closely related to fibrosis, and at the same time promote their differentiation into myofibroblasts, characterized by: high expression of α-SMA on the cell surface and synthesis of Increased capacity of the extracellular matrix.
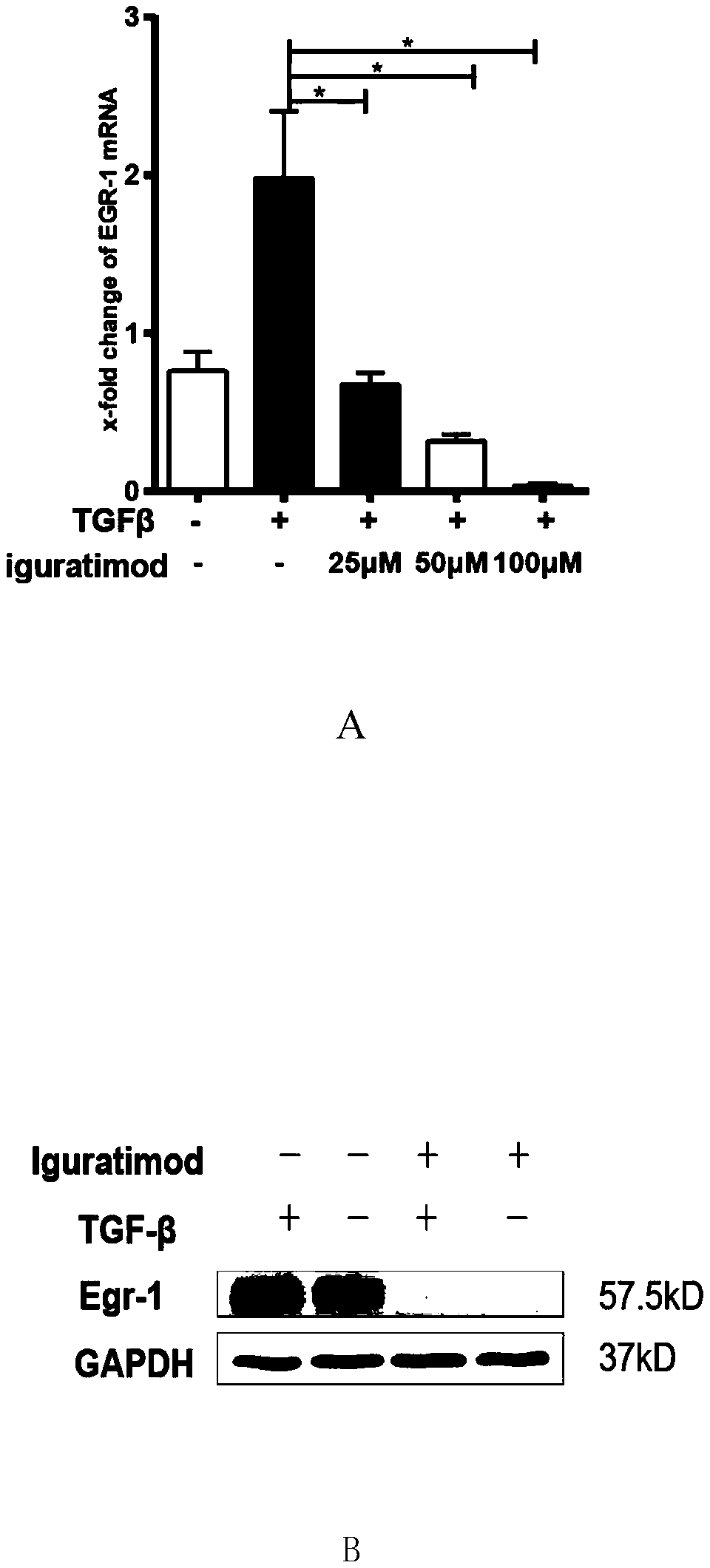
[0025] However, iguratimod can significantly inhibit the above-mentioned responses of fibroblasts at the mRNA and protein levels ( figure 1 , figure 2 ).
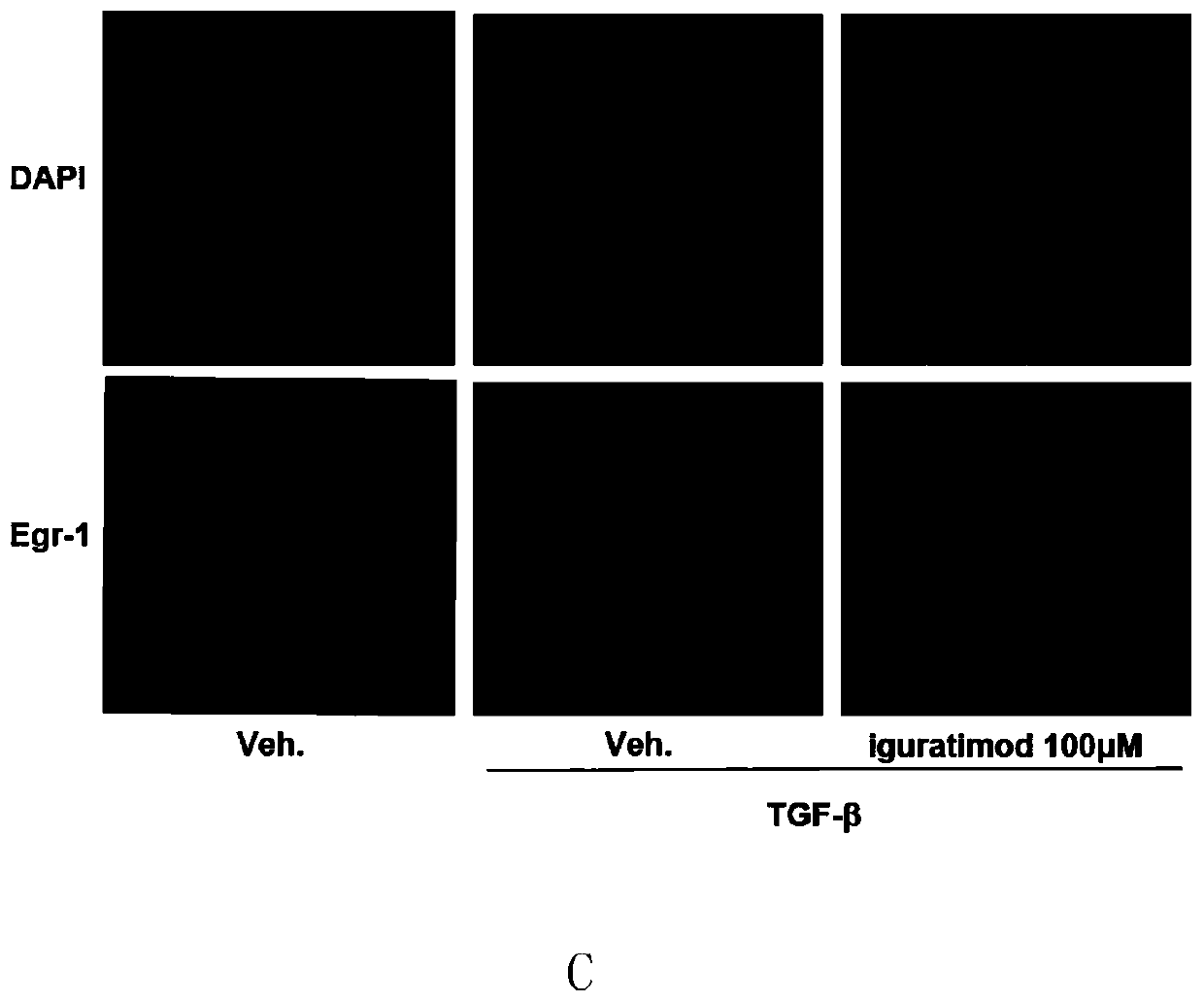
[0026] figure 1 Iguratimod was shown to inhibit TGF-β-induced expression of Egr-1 in healthy adult skin fibroblasts. (A) shows that iguratimod can inhibit the expression of Egr-1 induced by TGF-β at the mRNA level in a concentration-dependent manne...
Embodiment 2
[0029] In the present invention, male C57 / BL6 mice are subcutaneously injected with bleomycin to form a skin fibrosis model, and are divided into a control group 1 and a test group 2 after 2 weeks of modeling, and the test group is given iguratimod every day. Suspension 30mg / kg orally or apply 2% iguratimod dimethyl sulfoxide solution to the skin of the injection site twice a day, 100ul each time. Preparation of iguratimod suspension: 0.1% sodium carboxymethylcellulose (CMC) was used as suspending agent, and 60 mg of iguratimod was added to every 10 ml of suspending agent. Crush with a glass rod and shake well before use. Compared with the random control group, iguratimod intragastric administration and external application can significantly inhibit bleomycin-induced skin fibrosis ( image 3 A, C), and reduce the number of α-SMA-positive myofibroblasts in mouse skin ( image 3 B, D), and can effectively inhibit the expression of TGF-β and Egr1 in the skin. ( Figure 4 ). ...
Embodiment 3
[0035] 2% of the 6-week-old male TSK-1 mice of the present invention were given 100 μl of iguratimod DMSO solution for partial skin application, twice a day for 6 weeks in total. At the end of the 6th week, the mice were sacrificed, and the skin tissue was harvested, and the skin thickness was detected. The thickness of the subcutaneous fibrous tissue layer in TSK-1 mice was significantly greater than that in normal mice with isogenic background. After 6 weeks of experimental treatment, we found that the thickness of the subcutaneous fibrous layer of TSK-1 mice in the iguratimod smeared group decreased, and the difference was statistically significant (2110.3±110.3μm vs 1580±73.16μm P=0.0161) .
[0036] From this, we can think that iguratimod also has a therapeutic effect on a mouse model with severe fibrosis and mild inflammatory response that simulates the late stage of systemic sclerosis. Figure 5 showed that iguratimod topical application attenuated skin fibrosis in TSK...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com