Pharmaceutical composition used for treating ovarian cancer and application thereof
A composition and technology for ovarian cancer, applied in the field of treatment of ovarian cancer, can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of the pharmaceutical composition of anti-ovarian cancer, its steps are:
[0030] The active components of the pharmaceutical composition are kaempferol and tripterine, wherein the weight ratio of kaempferol to tripterine is 10:1, and the two drugs are prepared into solutions respectively, and then fully mixed evenly.
Embodiment 2
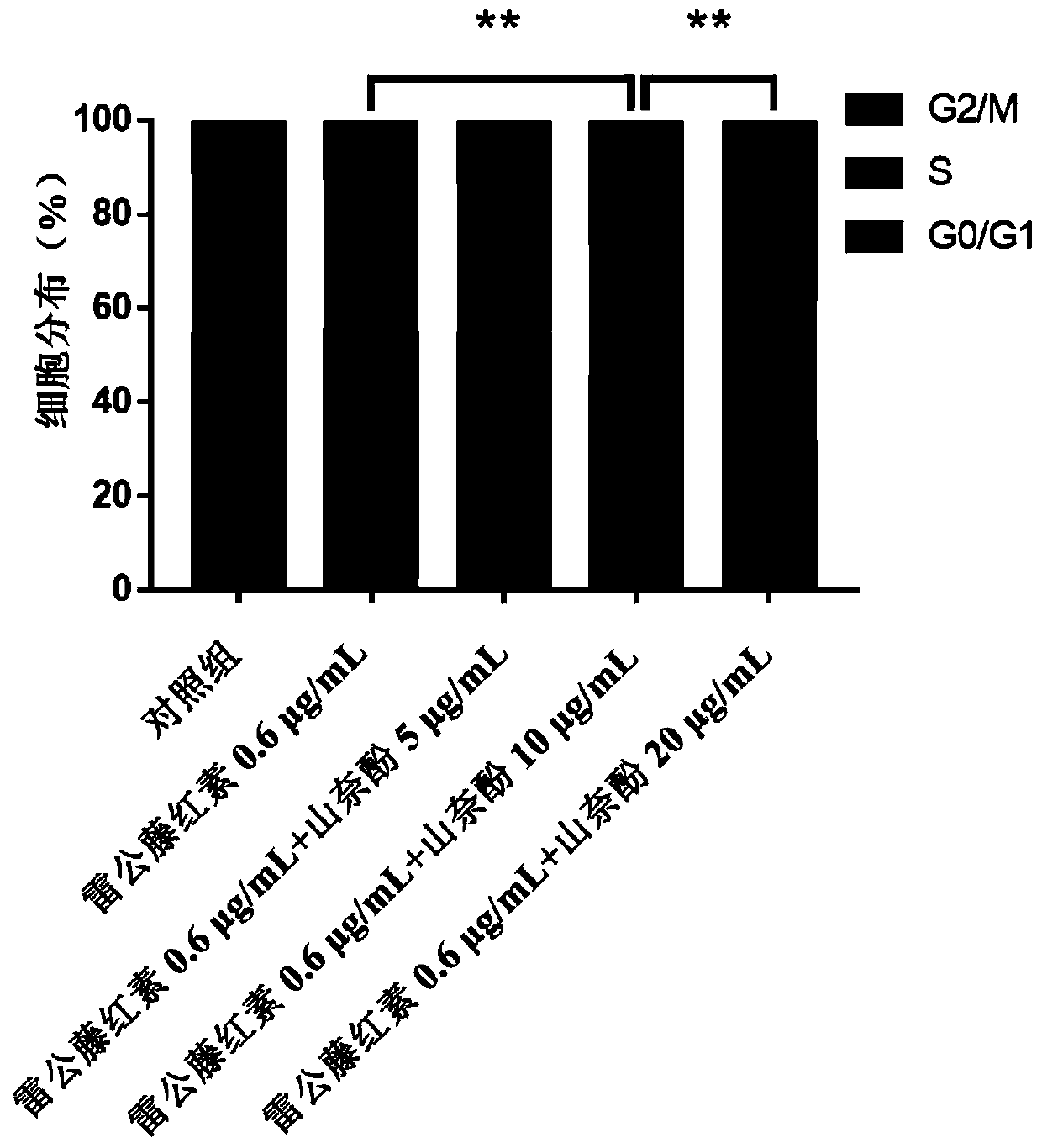
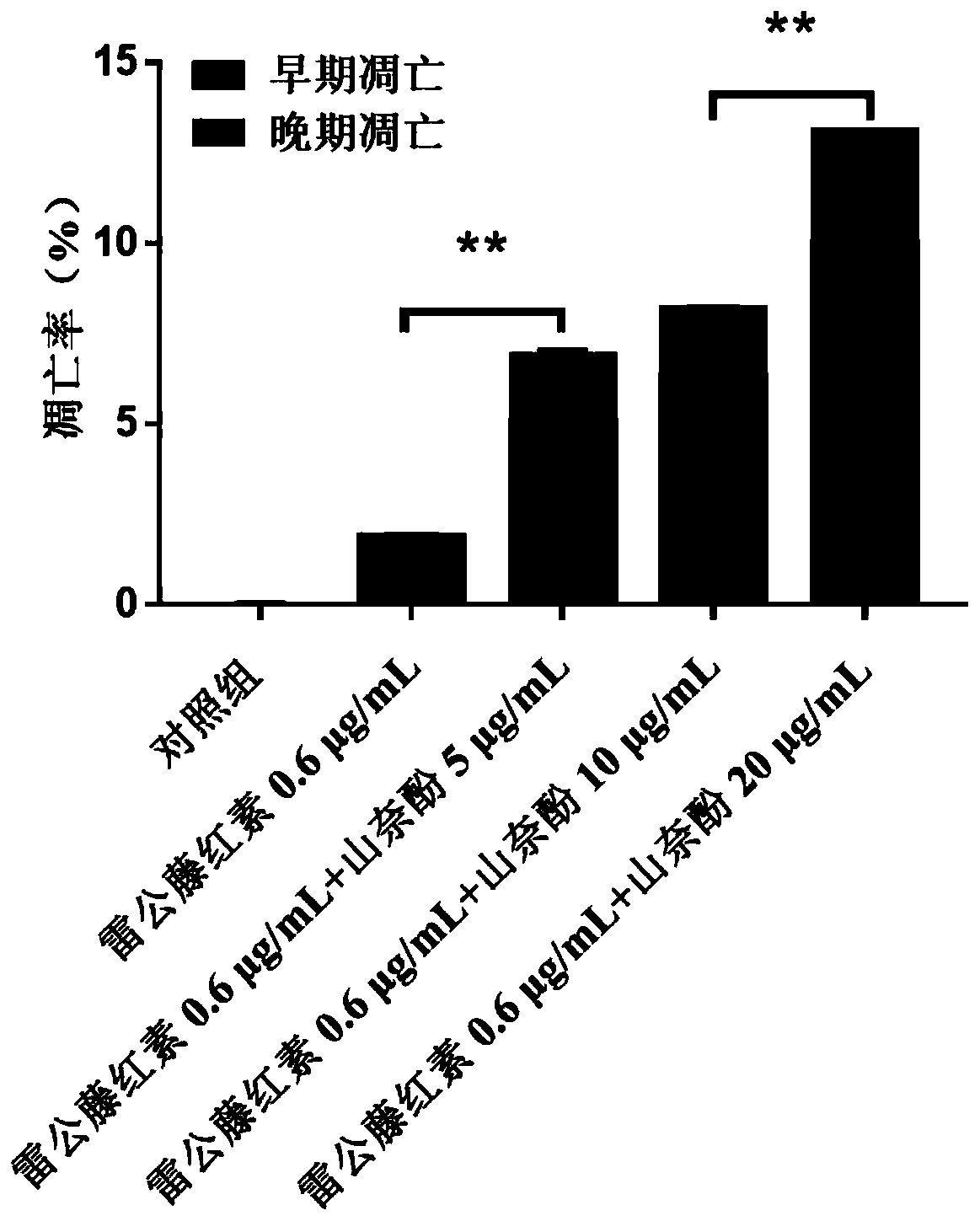
[0032] Application of the pharmaceutical composition against ovarian cancer:
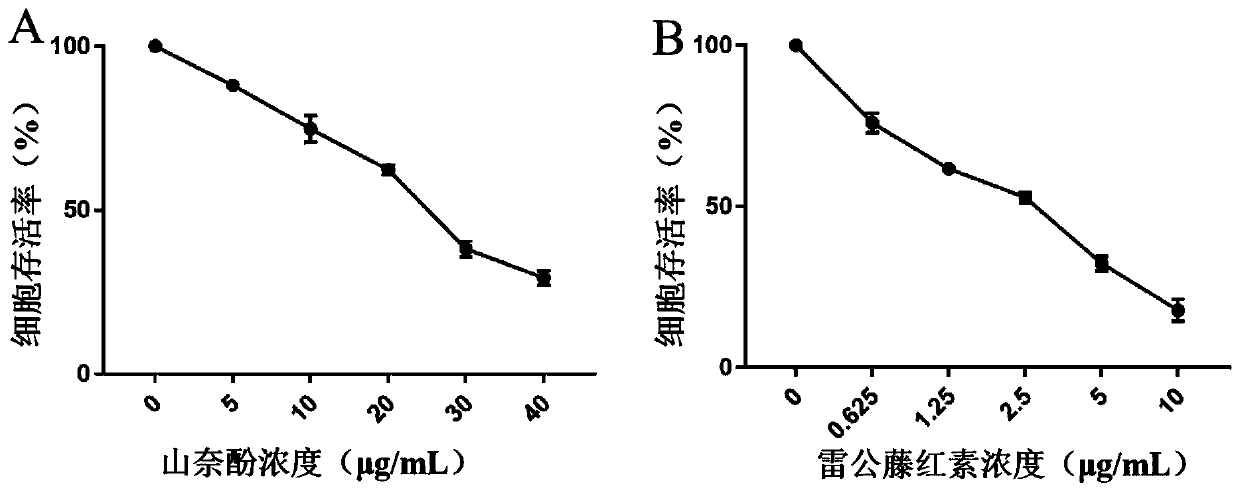
[0033] (1) Cytotoxicity test and drug interaction index evaluation
[0034] Human ovarian cancer cells A2780 were treated with 1×10 5 The density of cells / well was inoculated into 96-well plates, incubated for 24 hours, and then the cells were exposed to different concentrations of kaempferol (5-40 μg / mL) and tripterine (0.625-10 μg / mL) for 24 hours. The cells were then incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for 4 hours, and the formed formazan crystals were dissolved with 150 μL DMSO , after mixing with a micro-oscillator, use a microplate reader to measure the absorbance (OD) at 492nm, the experiment was repeated three times, and the average value was taken. The cell survival rate is equal to the ratio of the OD value of the administration group to the OD value of the blank group, that is, the cell survival rate=(OD 样品组 / OD 对照组 )×100%. Take the drug ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com