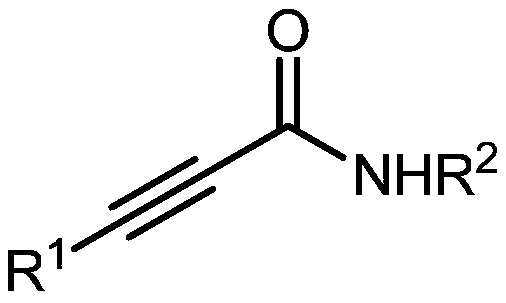
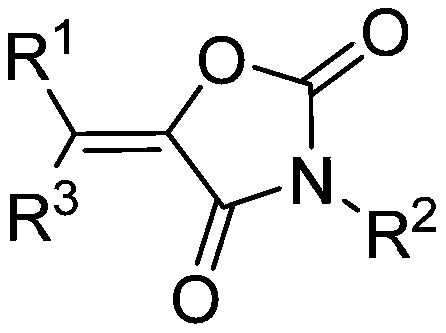
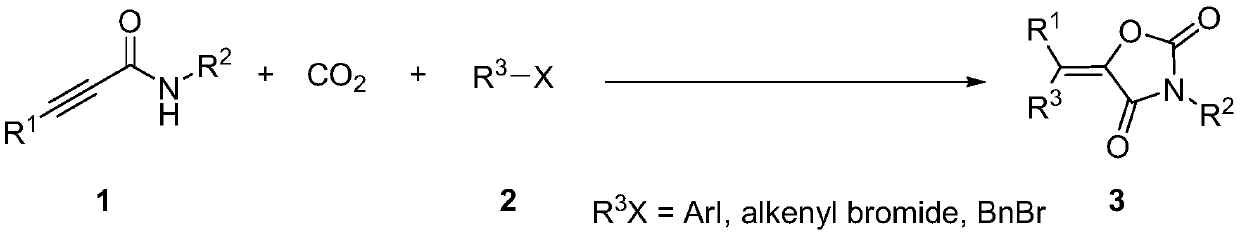Method for producing tetra-substituted vinyl 2,4-oxazolidinedione from carbon dioxide
A technology of vinyl oxazolidine and carbon dioxide, which is applied in the field of preparation of tetrasubstituted vinyl oxazolidine-2,4-dione, and functionalization reaction, which can solve the problems of expensive and difficult-to-obtain substrates, and achieve the extension of product types , mild reaction conditions and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 3-benzyl-5-(dibenzylidene)oxazolidine-2,4-dione
[0026] [3-benzyl-5-(diphenylmethylene)oxazolidine-2,4-dione]
[0027]
[0028] In the dried Schlenk tube, add in sequence: N-benzyl-3-phenylpropanamide (0.2mmol, 47mg), potassium carbonate (0.4mmol, 55.2mg), iodobenzene (0.24mmol, 49mg) , bistriphenylphosphine palladium dichloride (0.02mmol, 14mg), cuprous iodide (0.03mmol, 6mg), dimethyl sulfoxide 2mL, with CO 2 Replace the gas in the reaction tube three times, and finally fill the reaction tube with 0.1MPa CO 2 , and the above-mentioned Schlenk tube was placed at ambient temperature and stirred for 24 h. The reaction was terminated, and the reaction solution was quenched with 2 mL of saturated brine, extracted several times with ethyl acetate (4 mL×5), the organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatography (eluent: ethyl acetate: petroleum ether ...
Embodiment 2
[0030] Example 2: Preparation of 3-benzyl-5-(dibenzylidene)oxazolidine-2,4-dione
[0031] [3-benzyl-5-(diphenylmethylene)oxazolidine-2,4-dione]
[0032]
[0033] In the dried Schlenk tube, add in sequence: N-benzyl-3-phenylpropanamide (0.2mmol, 47mg), potassium carbonate (0.4mmol, 55.2mg), iodobenzene (0.24mmol, 49mg) , bistriphenylphosphine palladium dichloride (0.01mmol, 7mg), cuprous iodide (0.06mmol, 6mg), dimethyl sulfoxide 2mL, with CO 2 Replace the gas in the reaction tube three times, and finally fill the reaction tube with 0.1MPa CO 2 , and the above-mentioned Schlenk tube was placed at ambient temperature and stirred for 24 h. The reaction was terminated, and the reaction solution was quenched with 2 mL of saturated brine, extracted several times with ethyl acetate (4 mL×5), the organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatography (eluent: ethyl acetate: petroleum ether =...
Embodiment 3
[0034] Example 3: Preparation of 3-benzyl-5-(dibenzylidene)oxazolidine-2,4-dione
[0035] [3-benzyl-5-(diphenylmethylene)oxazolidine-2,4-dione]
[0036]
[0037] In the dried Schlenk tube, add in sequence: N-benzyl-3-phenylpropanamide (0.2mmol, 47mg), potassium carbonate (0.4mmol, 55.2mg), iodobenzene (0.24mmol, 49mg) , bistriphenylphosphine palladium dichloride (0.015mmol, 10.5mg), cuprous iodide (0.05mmol, 9.5mg), dimethyl sulfoxide 2mL, with CO 2 Replace the gas in the reaction tube three times, and finally fill the reaction tube with 0.1MPa CO 2 , and the above-mentioned Schlenk tube was placed at ambient temperature and stirred for 24 h. The reaction was terminated, and the reaction solution was quenched with 2 mL of saturated brine, extracted several times with ethyl acetate (4 mL×5), the organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatography (eluent: ethyl acetate: petroleum e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com