Aryl triazene compound preparation method
A technology for aryl triazene and compound, which is applied in the field of preparation of aryl triazene compounds, namely 1--3-triaza-1-ene, can solve the problems of difficulty in recovery, expensive catalyst and the like, and achieves a high price. Inexpensive, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 (the influence of diazotization process temperature on yield)
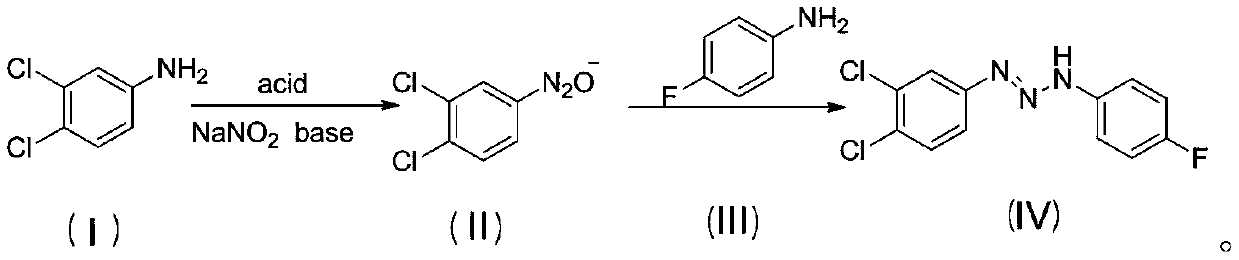
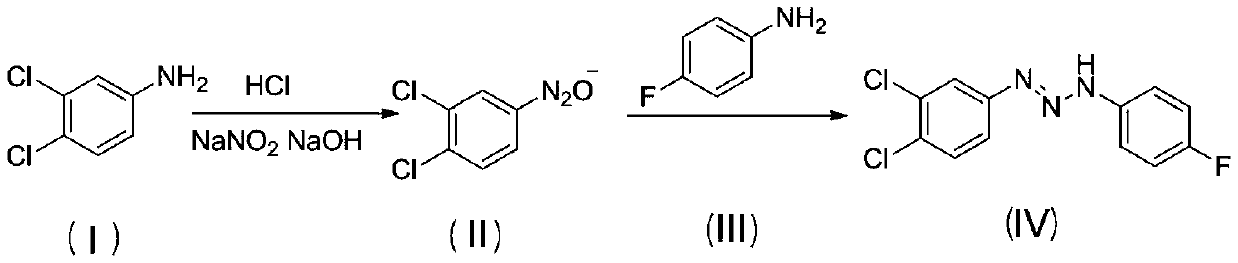
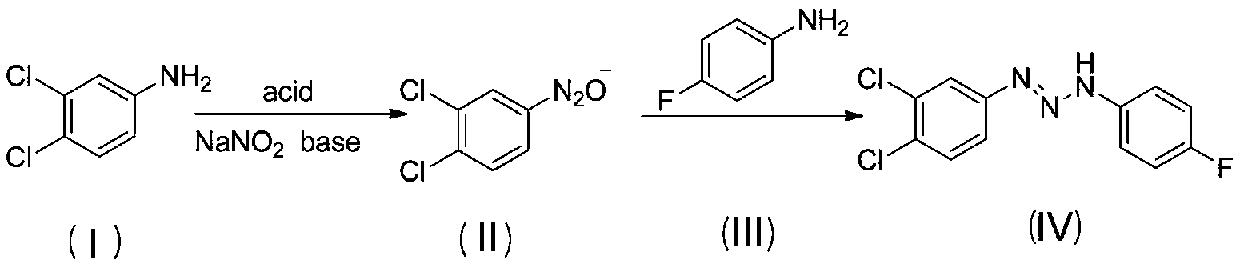
[0024] Add 1.36g (0.01mmol) of 3,4-dichloroaniline and 1mL of distilled water into a 50mL flask, heat to 75°C, add 5.5mL of concentrated hydrochloric acid with a mass fraction of 36% dropwise after it dissolves, add 5mL of distilled water after stirring vigorously, Heat and stir intermittently until it is completely dissolved, add 15mL of water after natural cooling to room temperature, stir to form a uniform white milky suspension, place the flask in an ice-water bath at -5 to 5°C, and add sodium nitrite aqueous solution dropwise (5 mL, 0.0105 mol, 2.1 M), and continued to stir for 2 h to obtain an aqueous solution of 3,4-dichloroaniline diazonium salt.
[0025] Add aqueous sodium hydroxide solution (15mL, 0.06mol, 4M) into a 100mL beaker, bathe in ice water, and when the solution drops to -5~5°C, slowly add 3,4-dichloroaniline dropwise within 30min under vigorous stirring For the diazonium s...
Embodiment 2
[0032] Embodiment 2 (the influence of the mol ratio of substituted aniline and sodium nitrite on productive rate)
[0033] Add 1.36g (0.01mol) of 3,4-dichloroaniline and 1mL of distilled water into a 50mL flask, heat to 75°C, and after it dissolves, add 5.5mL of concentrated hydrochloric acid with a mass fraction of 36%, add 5mL of distilled water after vigorous stirring , heated and stirred intermittently until it was completely dissolved, cooled naturally to room temperature, added 15 mL of water, stirred to form a uniform white milky suspension, placed the flask in an ice-water bath at 0-5°C, repeated the above experiment three times, and Add dropwise 5.0mL, 5.7mL, 6.2mL of 2.1M sodium nitrite aqueous solution (that is, the amount of sodium nitrite is 0.0105, 0.012, 0.013mol respectively), and continue to stir for 2h to obtain three parts of 3,4-di Chloroaniline diazonium salt solution, and coded as a, b, c;
[0034] Take three 100mL beakers and add aqueous sodium hydroxid...
Embodiment 3
[0042] Embodiment 3 (the influence of reactant (Ⅲ) preheating temperature on yield)
[0043]Add 1.36g (0.01mmol) of 3,4-dichloroaniline and 1mL of distilled water into a 50mL flask, heat to 75°C, add 5.5mL of concentrated hydrochloric acid with a mass fraction of 36% dropwise after it dissolves, add 5mL of distilled water after stirring vigorously, Heat and stir intermittently until it dissolves completely, add 15mL of water after natural cooling to room temperature, stir to form a uniform white milky suspension, place the flask in an ice-water bath at 0-5°C, add sodium nitrite aqueous solution ( 5mL, 0.0105mol, 2.1M), and continued stirring for 2h to obtain an aqueous solution of 3,4-dichloroaniline diazonium salt.
[0044] Add aqueous sodium hydroxide solution (15mL, 0.06mol, 4M) into a 100mL beaker, take an ice-water bath, and when the solution drops to 0-5°C, slowly add 3,4-dichloroaniline weight dropwise within 30min under vigorous stirring. Nitrogen salt solution, after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com