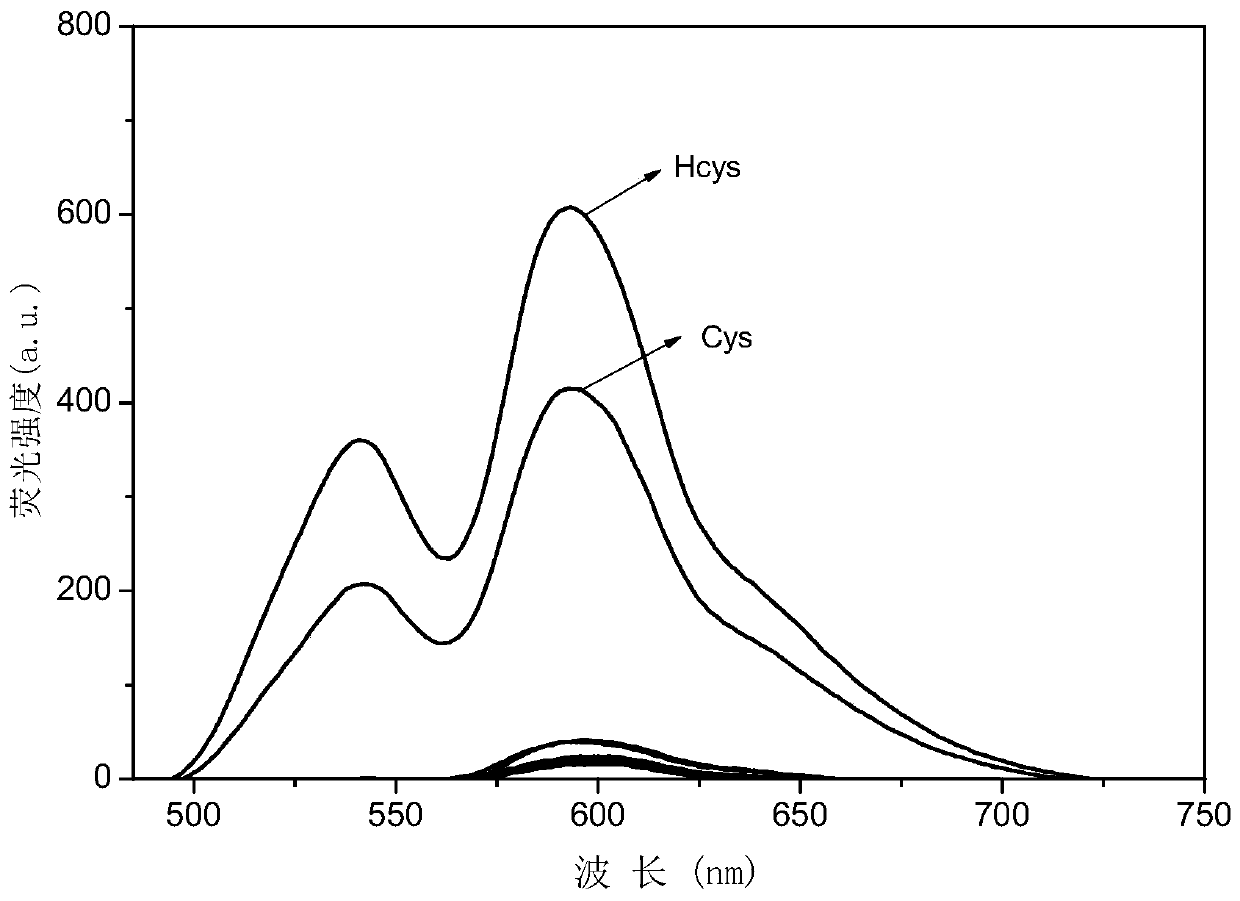
Fluorescent probe based on 2-styryl indole salt type derivative long-wave emission for distinguishable detection on Cys/Hcy and application of florescent probe
A fluorescent probe and styryl technology, which is applied in the distinguishable detection of Cys/Hcy fluorescent probe based on long-wave emission of 2-styryl indole salt derivatives and its application field, which can solve the problem of inability to distinguish and identify Cys/Hcy , the synthesis route is complex, and the rapid detection is not possible, so as to achieve the effect of easy separation and purification, simple synthesis process and good sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The specific synthesis steps of fluorescent probe L are as follows:
[0034]
[0035] Compound 1 (487.17 mg, 1 mmol), 4-chloro-7-nitrobenzo-2-oxa-1,3-oxadiazole (139.46 mg, 1.2 mmol), triethylamine (101 mg, 0.1 mmol) were dissolved Yu Qian CH 2 Cl 2 (20 mL), stirred at room temperature for 6 hours. Then water was added, the mixture was extracted with ethyl acetate, washed with anhydrous Na 2 SO 4 The organic layer was dried, the solvent was removed, and the crude product was purified by column chromatography using methanol / dichloromethane (1:50, v / v) as eluent to obtain compound L (184 mg, 34.9%). 1 H NMR (400MHz, DMSO-d 6 )δ8.69(d, J=8.3Hz, 1H), 8.40(d, J=9.2Hz, 1H), 8.13(d, J=15.7Hz, 1H), 7.73(t, J=7.5Hz, 2H) ,7.54(td,J=7.7,1.3Hz,1H),7.46(t,J=7.5Hz,1H),7.36(d,J=15.7Hz,1H),6.98(dd,J=9.2,2.4Hz, 2H), 6.95(d, J=2.4Hz, 1H), 4.44(q, J=7.2Hz, 2H), 3.56(q, J=7.0Hz, 4H), 1.46(s, 6H), 1.30(t, J=7.2Hz, 3H), 1.17(t, J=7.0Hz, 6H).
[0036]13 C NMR (101MHz, DMSO-d 6 )δ...
Embodiment 2
[0039] The specific synthesis steps of fluorescent probe L are as follows:
[0040] Compound 1 (487.17 mg, 1 mmol), 4-chloro-7-nitrobenzo-2-oxa-1,3-oxadiazole (174.32 mg, 1.5 mmol), triethylamine (303 mg, 0.3 mmol) were dissolved Yu Qian CH 2 Cl 2 (20 mL), stirred at room temperature for 9 hours. Then water was added, the mixture was extracted with ethyl acetate, washed with anhydrous Na 2 SO 4 The organic layer was dried, the solvent was removed, and the crude product was purified by column chromatography using methanol / dichloromethane (1:80, v / v) as eluent to obtain compound L (150 mg, 28.6%). Fluorescent probe L of this embodiment 1 H NMR spectrum and 13 C NMR spectrum as figure 1 and figure 2 . HRMS (ESI+) Calcd for C 30 h 32 N 5 o 4 [M-I] + :526.2449,found:526.2713.
Embodiment 3
[0042] The specific synthesis steps of fluorescent probe L are as follows:
[0043] Compound 1 (487.17 mg, 1 mmol), 2 4-chloro-7-nitrobenzo-2-oxa-1,3-oxadiazole (232.43 mg, 2 mmol), triethylamine (505 mg, 0.5 mmol) were dissolved Yu Qian CH 2 Cl 2 (20 mL), the mixture was stirred at room temperature for 12 hours. Then water was added, the mixture was extracted with ethyl acetate, washed with anhydrous Na 2 SO 4 The organic layer was dried, the solvent was removed, and the crude product was purified by column chromatography using methanol / dichloromethane (1:100, v / v) as eluent to obtain compound L (113 mg, 21.5%). Fluorescent probe L of this embodiment 1 H NMR spectrum and 13 C NMR spectrum as figure 1 and figure 2 . HRMS (ESI+) Calcd for C 30 h 32 N 5 o 4 [M-I] + :526.2449,found:526.2713.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com