Fluazinam hapten, artificial antigen and antibody and preparation method and application thereof
An artificial antigen, fluazinamide technology, applied in the preparation method of peptide, chemical instrument and method, animal/human protein, etc., to achieve the effect of enhanced immunogenicity, simple reaction operation and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
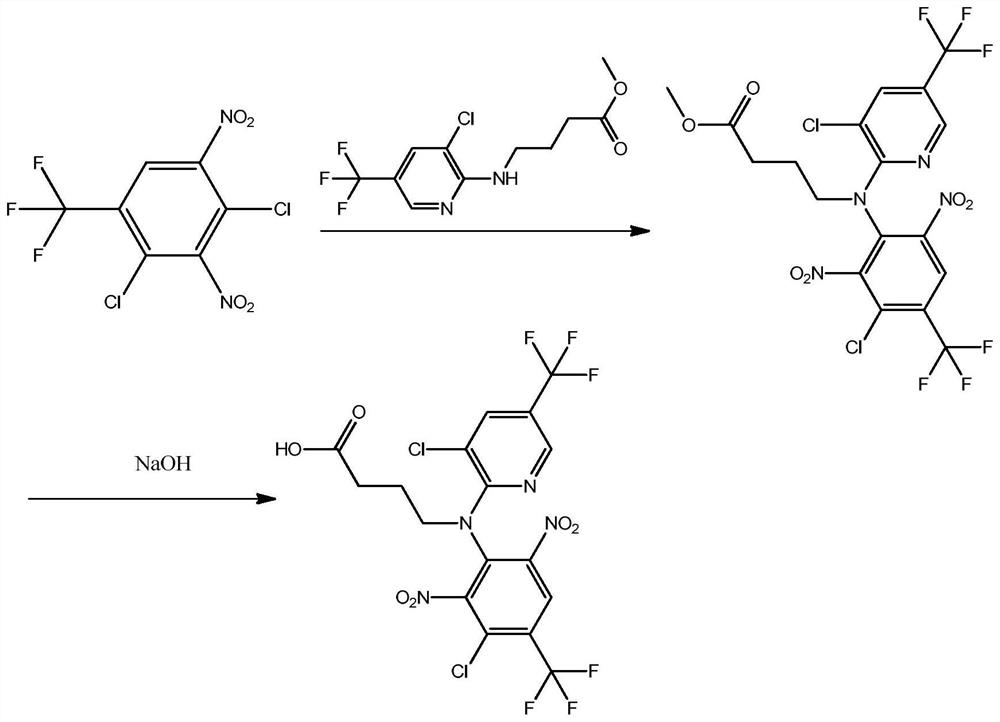
[0029] In a second aspect, the present invention provides a method for preparing the above-mentioned fluazinam hapten, comprising the following steps:
[0030] 1) 2,4-dichloro-3,5-dinitrotrifluorotoluene reacts with methyl 2-aminobutyrate-3-chloro-5-trifluoromethylpyridine to obtain intermediate 1, the intermediate Body 1 has the structural formula
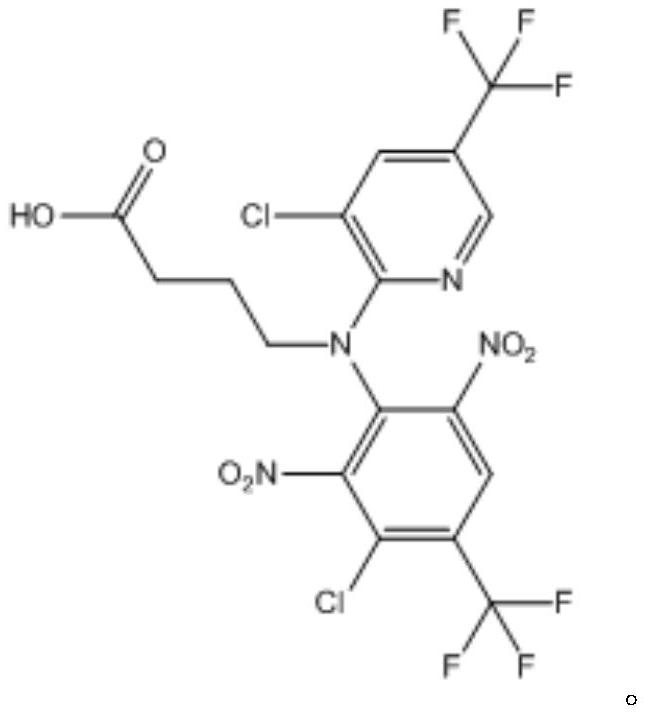
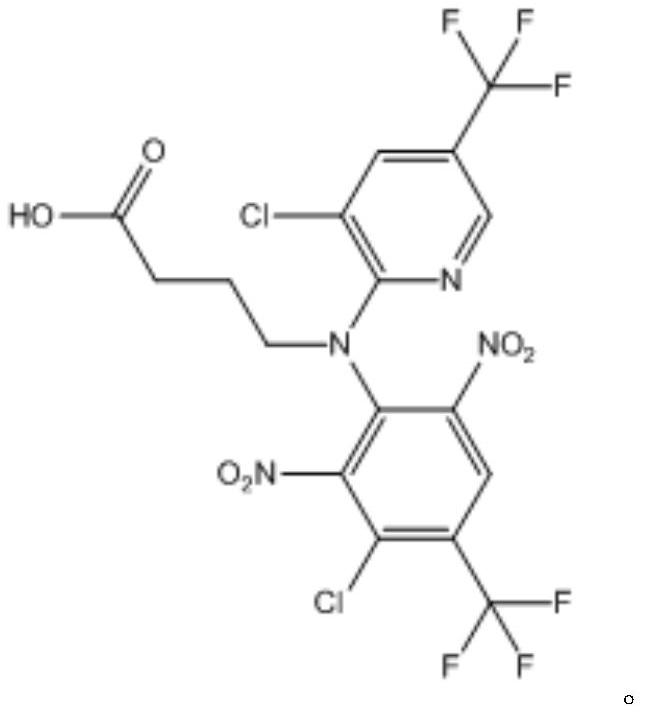
[0031] 2) The intermediate 1 is subjected to a hydrolysis reaction to obtain the fluazinam hapten.
[0032] Preferably, the step 1) includes the following steps: taking 2,4-dichloro-3,5-dinitrotrifluorotoluene and adding an organic solvent to dissolve, adding anhydrous potassium carbonate and 2-aminobutyric acid methyl ester-3 -Chloro-5-trifluoromethylpyridine, heated in an oil bath for reaction, after the reaction was completed, rotary evaporated, added water, extracted with ethyl acetate, collected the organic phase, evaporated the organic phase to dryness, and then used dichloromethane-cyclohexane beating with alkane, sucti...
Embodiment 1
[0051] A kind of preparation method of fluazinam hapten, it comprises the steps:
[0052] 1) Dissolve 0.304g of 2,4-dichloro-3,5-dinitrotrifluorotoluene in 80mL of acetonitrile, add 0.32g of anhydrous potassium carbonate and 0.21g of methyl 2-aminobutyrate-3-chloro-5 -Trifluoromethylpyridine, heated in an oil bath for 12h, after the reaction was completed, the acetonitrile was removed by rotary evaporation, 60mL of water was added, extracted with 80mL of ethyl acetate, the organic phase was collected, and the organic phase was evaporated to dryness with 20mL volume ratio of 1 : 3 of dichloromethane-cyclohexane was slurried, filtered with suction, and washed with n-hexane to obtain Intermediate 1;
[0053] 2) Weigh intermediate 1, add alkaline solution, heat to reflux, and carry out hydrolysis reaction; after the reaction, adjust pH to slightly acidic with hydrochloric acid, then extract with ethyl acetate, and the organic phase is evaporated to dryness and purified by column t...
Embodiment 2
[0055] A kind of preparation method of fluazinam hapten, it comprises the steps:
[0056] 1) Dissolve 0.304g of 2,4-dichloro-3,5-dinitrotrifluorotoluene in 80mL of acetonitrile, add 0.28g of anhydrous potassium carbonate and 0.17g of methyl 2-aminobutyrate-3-chloro-5 -Trifluoromethylpyridine, heated in an oil bath for 12h, after the reaction was completed, the acetonitrile was removed by rotary evaporation, 60mL of water was added, extracted with 80mL of ethyl acetate, the organic phase was collected, and the organic phase was evaporated to dryness with 20mL volume ratio of 1 : 3 of dichloromethane-cyclohexane was slurried, filtered with suction, and washed with n-hexane to obtain Intermediate 1;
[0057] 2) Weigh intermediate 1, add alkaline solution, heat to reflux, and carry out hydrolysis reaction; after the reaction, adjust pH to slightly acidic with hydrochloric acid, then extract with ethyl acetate, and the organic phase is evaporated to dryness and purified by column t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com