A controllable preparation method based on covalent organic framework and its application in energy storage
A covalent organic framework and mechanical technology, applied in the direction of hybrid capacitor electrodes, can solve the problems that the shape and size of COFs materials cannot be precisely controlled, and the advantages of microstructure cannot be fully utilized, so as to improve the shape of the amorphous and growth process. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A controllable preparation method based on a covalent organic framework, adding 0.42 g (0.2 mmol) of trihydroxybenzenetricarbaldehyde and 0.65 g (0.3 mmol) of 2,6-diaminoanthraquinone into an agate mortar and grinding Add 6 μL of a mixed solvent of mesitylene and dioxane (1:1) and 0.1 μL of 6 mol / L acetic acid as a catalyst dropwise until the powder turns orange-red. Add the obtained orange-red powder into an ampoule (capacity about 25 mL, tube length 20 cm) containing 12 mL of mesitylene and dioxane (1:1) solution, ultrasonically disperse the mixture evenly, and add 0.6 mL of 6 mol / L acetic acid solution. Then the system was frozen with liquid nitrogen, the tube was sealed and evacuated, sealed with a flame spray gun and placed in an oven at 120 °C for 96 h to ensure that there was no interference. After the oven was cooled to room temperature, the ampoule was taken out, and the obtained product was washed repeatedly with tetrahydrofuran and dichloromethane, respectiv...
Embodiment 2
[0067] A controllable preparation method based on covalent organic framework, adding 0.42 g (0.2 mmol) of trihydroxybenzenetricarbaldehyde and 0.65 g (0.3 mmol) of 2,6-diaminoanthraquinone into a 12 mL mesitylene and dioxane (1:1) solution in an ampoule (capacity about 25 mL, tube length 20 cm), the mixture was ultrasonically dispersed, and 0.6 mL of 6 mol / L acetic acid solution was added dropwise. Then the system was frozen with liquid nitrogen, the tube was sealed and evacuated, sealed with a flame spray gun and placed in an oven at 120 °C for 96 h to ensure that there was no interference. After the oven was cooled to room temperature, the ampoule was taken out, and the obtained product was washed repeatedly with tetrahydrofuran and dichloromethane, and dried in a vacuum oven at 85°C for 12 h to obtain a C-S-COFs electrode material with a simulated flower shape.
[0068] Carry out performance test and characterization to embodiment 2 gained product:
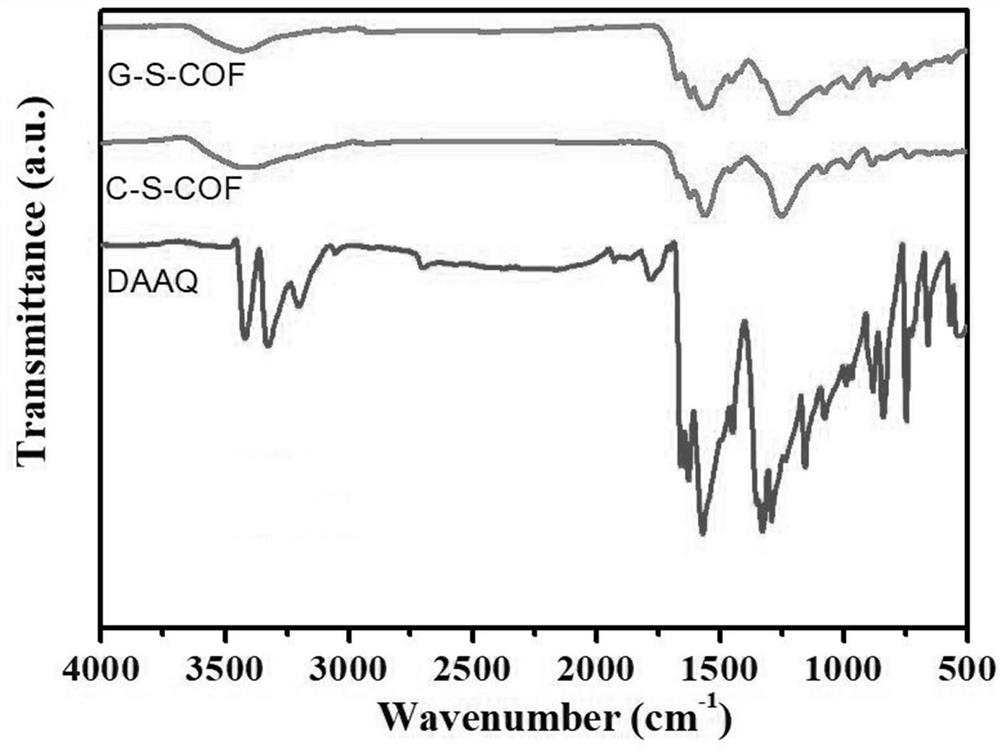
[0069] 1. Infrared spe...
Embodiment 3-5
[0084] Except that the polymerization solvent was different, other conditions were the same as in Example 2, and the influence of the polarity of the polymerization solvent on COFs was explored.
[0085] Add 0.42 g (0.2 mmol) of trihydroxybenzenetricarbaldehyde and 0.65 g (0.3 mmol) of 2,6-diaminoanthraquinone into an ampoule containing 12 mL of mesitylene solution (capacity about 25 mL, tube length 20 cm), the mixture was ultrasonically dispersed, and 0.6 mL of 6 mol / L acetic acid solution was added dropwise. Then the system was frozen with liquid nitrogen, the tube was sealed and evacuated, sealed with a flame spray gun and placed in an oven at 120 °C for 24 h without any disturbance. After the oven was cooled to room temperature, the ampoule was taken out, and the obtained product (M-COFs) was washed repeatedly with tetrahydrofuran and dichloromethane, respectively, and dried in a vacuum oven at 85 °C for 12 h. Such as Figure 6 As shown, M-COFs can be seen assembled into...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com