P, N and Si ternary hybrid flame retardant and preparation method thereof
A flame retardant, ternary hybrid technology, applied in the field of P, N, Si ternary hybrid flame retardant and preparation, can solve the problems of cumbersome and harsh preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
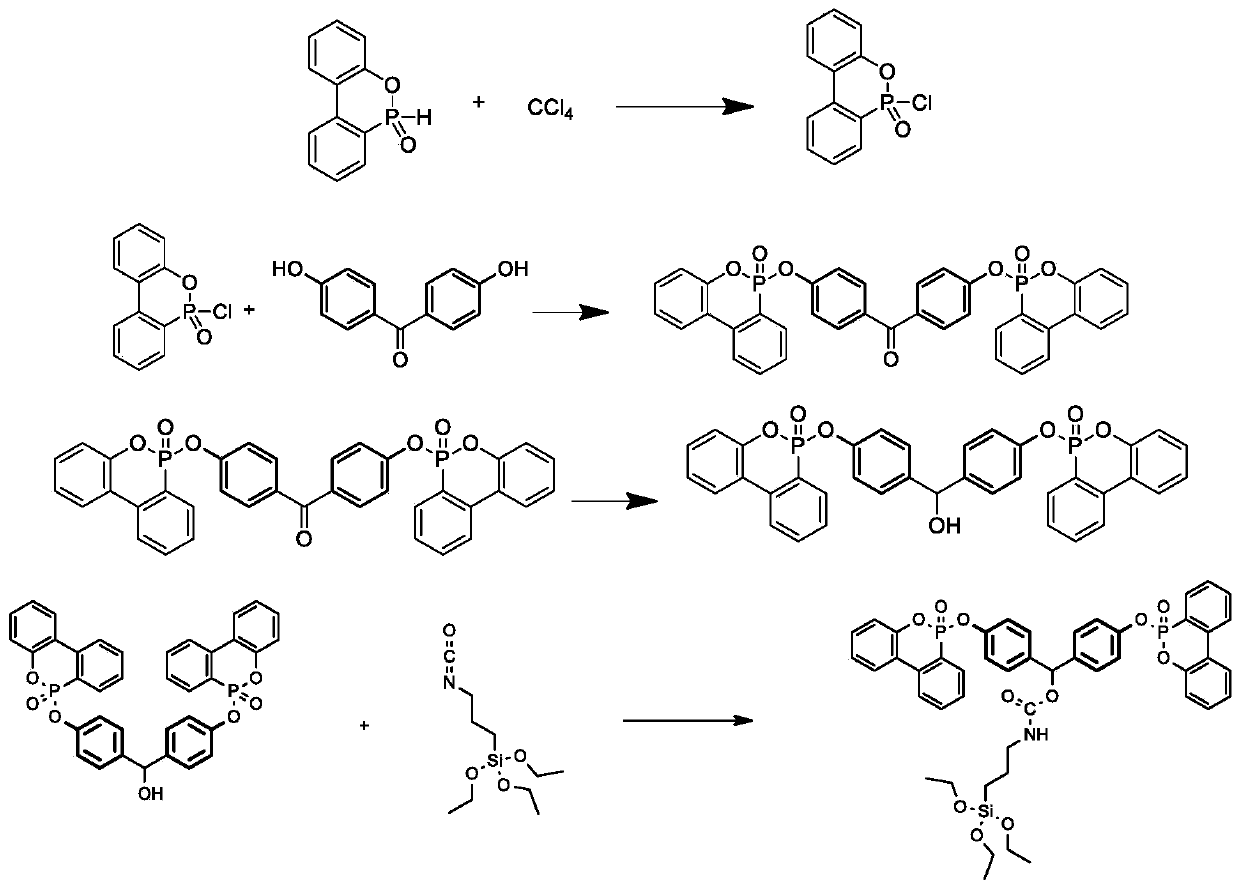
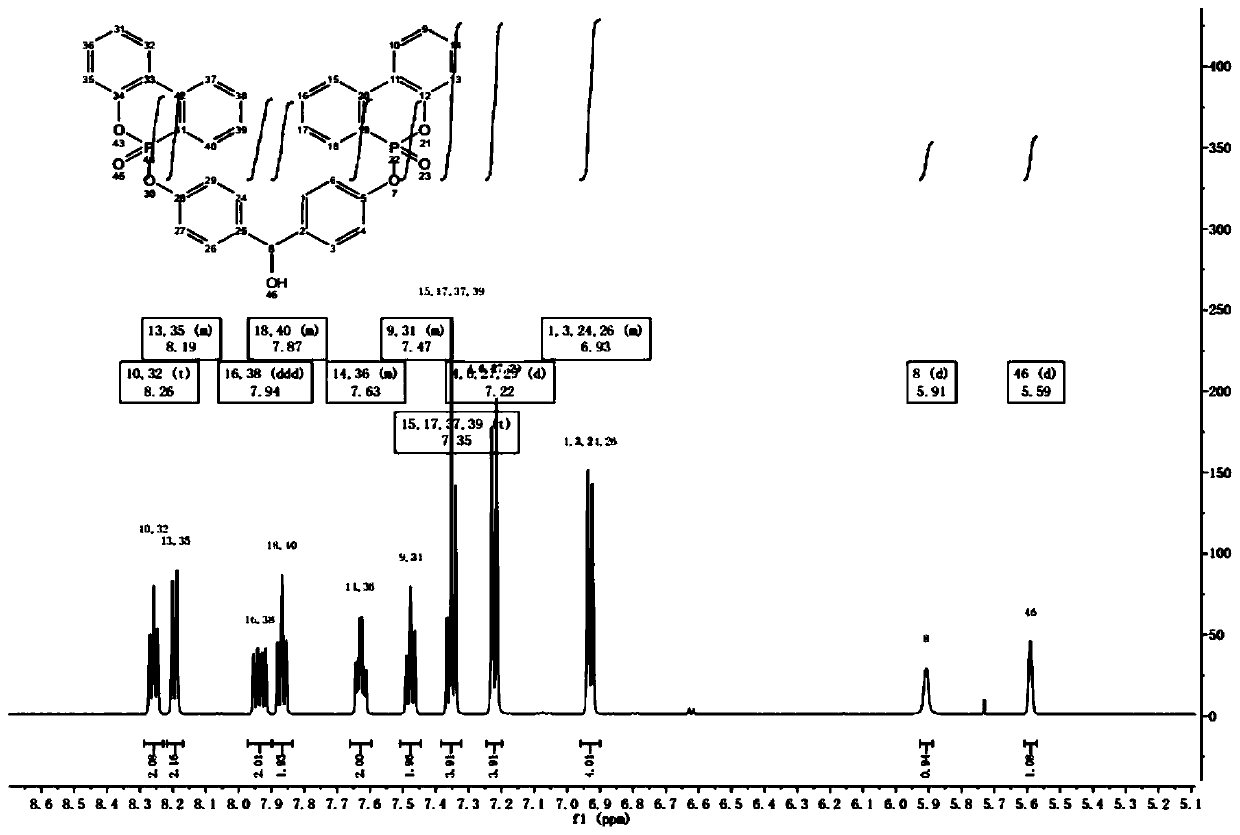
[0033] Add 1.0mol 4,4'-dihydroxybenzophenone (DHBP), dichloromethane, and 3.0mol triethylamine into a reaction vessel equipped with stirring, heating, and temperature control devices; stir for 30 minutes under nitrogen protection Add 2.0mol 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and 2.0mol carbon tetrachloride (CCl 4 ), stirring and reacting at 25°C for 1h after the addition was completed, adding water to quench the reaction, extracting 3 times with dichloromethane, washing the organic phase 3 times with deionized water, evaporating the dichloromethane to dryness under reduced pressure to obtain a white solid, which was purified by column chromatography The product was separated and purified to obtain 4,4'-bis(diphenoxyphosphor-6-oxyl)benzophenone (DODH). Then add 1.0mol DODH, tetrahydrofuran, 1.0mol NaBH into the reaction vessel equipped with stirring, heating and temperature control devices 4 ; Stir at 25°C for 4h after the addition, evaporate the solvent...
Embodiment 2
[0035]Add 1.0mol 4,4'-dihydroxybenzophenone (DHBP), dichloromethane, and 4.6mol triethylamine into a reaction vessel equipped with stirring, heating, and temperature control devices; stir for 30 minutes under nitrogen protection Add 2.3mol 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and 3.0mol carbon tetrachloride (CCl 4 ), stirring and reacting at 25°C for 1h after the addition was completed, adding water to quench the reaction, extracting 3 times with dichloromethane, washing the organic phase 3 times with deionized water, evaporating the dichloromethane to dryness under reduced pressure to obtain a white solid, which was purified by column chromatography The product was separated and purified to obtain 4,4'-bis(diphenoxyphosphor-6-oxyl)benzophenone (DODH). Then add 1.0mol DODH, tetrahydrofuran, 1.3mol NaBH into the reaction vessel equipped with stirring, heating and temperature control devices 4 ; Stir at 25°C for 4h after the addition, evaporate the solvent ...
Embodiment 3
[0037] Add 1.0mol 4,4'-dihydroxybenzophenone (DHBP), dichloromethane, and 3.76mol triethylamine into a reaction vessel equipped with stirring, heating, and temperature control devices; stir for 30 minutes under nitrogen protection Add 2.15mol 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and 2.47mol carbon tetrachloride (CCl 4 ), stirring and reacting at 25°C for 1h after the addition was completed, adding water to quench the reaction, extracting 3 times with dichloromethane, washing the organic phase 3 times with deionized water, evaporating the dichloromethane to dryness under reduced pressure to obtain a white solid, which was purified by column chromatography The product was separated and purified to obtain 4,4'-bis(diphenoxyphosphor-6-oxyl)benzophenone (DODH). Then add 1.0mol DODH, tetrahydrofuran, 1.3mol NaBH into the reaction vessel equipped with stirring, heating and temperature control devices 4 ; Stir at 25°C for 4h after the addition, evaporate the solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com