Arylquinoline derivative synthesis method
An arylquinoline and a synthesis method technology, applied in the direction of organic chemistry and the like, can solve the problems of not conforming to the development of green chemistry, harsh reaction temperature and conditions, low atom utilization rate, etc., achieving easy commercial purchase or preparation, convenient operation, The effect of improved substrate availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
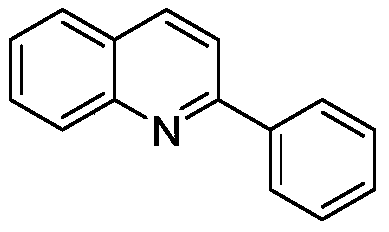
[0026] The preparation of 2-phenylquinoline, structural formula is as follows:
[0027]
[0028] Under air atmosphere, add raw material (E)-3-(2-aminobenzene)acrylonitrile (0.3mmol), phenylboronic acid (0.6mmol) and catalyst palladium trifluoroacetate (5mol%), p-toluenesulfonic acid monohydrate Compound (0.6mmol), toluene (2mL), reacted at 90°C for 36h, and the isolated yield of the product was 90%. 1 H NMR (500MHz, CDCl 3 )δ8.24-8.17(m,4H),7.89(d,J=11.0Hz,1H),7.84(d,J=10.0Hz,1H),7.76-7.72(m,1H),7.56-7.52(m ,3H),7.49-7.46(m,1H); 13 C NMR (125MHz, CDCl 3 )δ157.4, 148.3, 139.6, 136.8, 129.7, 129.6, 129.4, 128.8, 127.6, 127.4, 127.2, 126.3, 119.0.
Embodiment 2
[0030] The preparation of 2-(4-methylphenyl) quinoline, structural formula is as follows:
[0031]
[0032] Under air atmosphere, add raw material (E)-3-(2-anilino)acrylonitrile (0.3mmol), 4-methylphenylboronic acid (0.6mmol) and catalyst palladium trifluoroacetate (5mol%), p-toluene Sulfonic acid monohydrate (0.6mmol), toluene (2mL), reacted at 90°C for 36h, and the isolated yield of the product was 88%. 1 H NMR (500MHz, CDCl3) δ8.19-8.07(m, 4H), 7.87-7.81(m, 2H), 7.72-7.71(m, 1H), 7.51-7.48(m, 1H), 7.34-7.33(m ,2H),2.43(s,3H); 13 C NMR (125MHz, CDCl3) δ157.3, 148.2, 139.5, 136.8, 136.7, 129.7, 129.6, 129.5, 127.4, 127.1, 126.1, 118.9, 21.3.
Embodiment 3
[0034] The preparation of 2-(3,5-dimethylphenyl) quinoline, the structural formula is as follows:
[0035]
[0036] Under air atmosphere, add raw materials (E)-3-(2-anilino)acrylonitrile (0.3mmol), p-toluenesulfonic acid monohydrate (0.6mmol), 3,5-dimethylphenylboronic acid (0.6 mmol) and catalyst palladium trifluoroacetate (5mol%), toluene (2mL), reacted at 90°C for 36h, and the isolated yield of the product was 88%. 1 H NMR (500MHz, CDCl 3 )δ8.20(t, J=7.5Hz, 2H), 7.86(d, J=8.5Hz, 1H), 7.82(d, J=8.5Hz, 1H), 7.78(s, 2H), 7.75-7.72( m,1H),7.52(t,J=7.5Hz,1H),7.12(s,1H),2.45(s,6H); 13 C NMR (125MHz, CDCl 3 )δ157.8, 148.3, 139.7, 138.4, 136.6, 131.1, 129.7, 129.6, 127.5, 127.2, 126.2, 125.5, 119.3, 21.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com