Application berberine (BBR) in regulating functions of KCNH6 protein
A technology of berberine and protein, applied in the field of molecular biology, can solve the problem that molecular targets are poorly understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
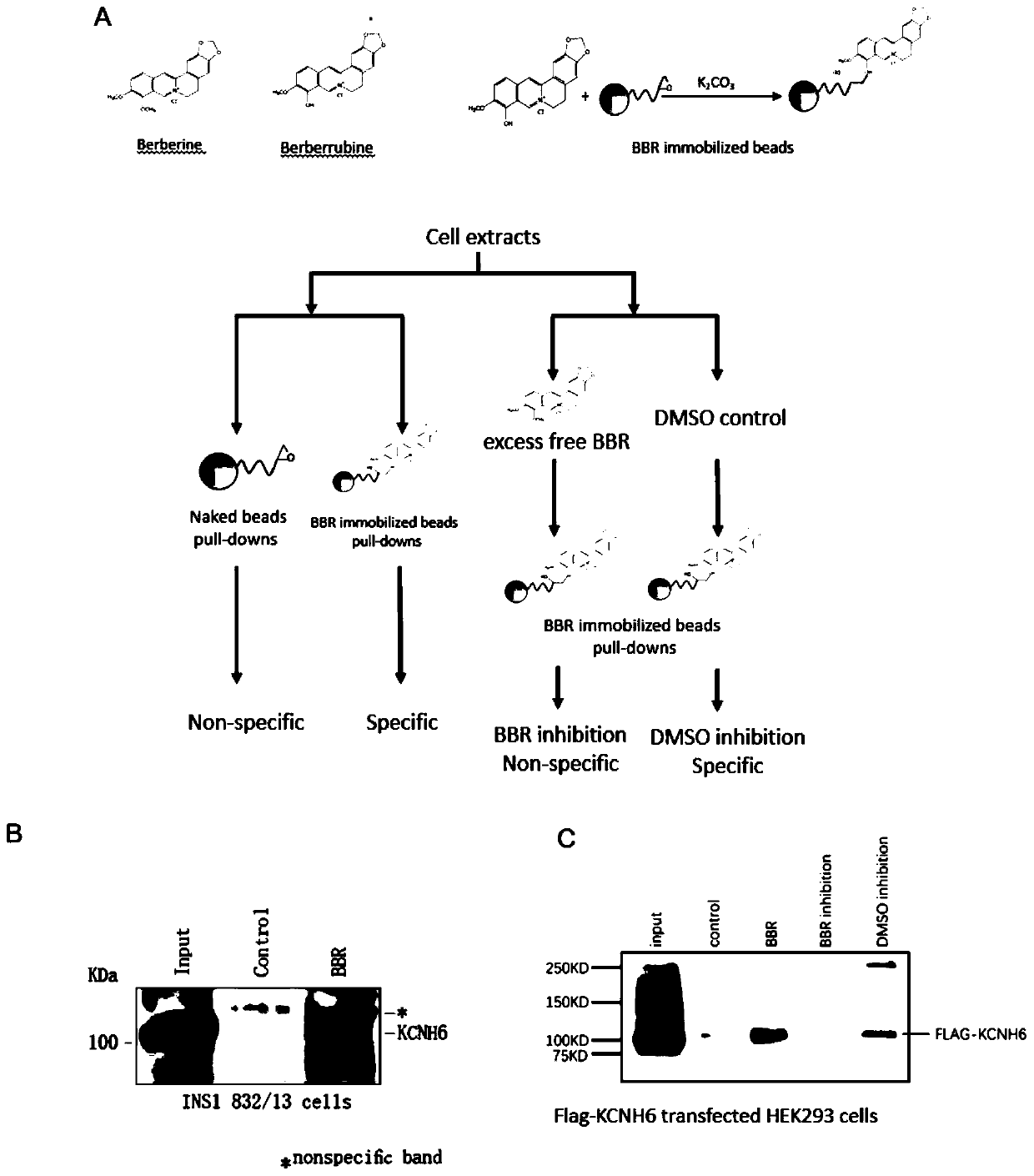
[0037] Preparation of BBR immobilized magnetic beads:
[0038] Fixed programs such as figure 2 As shown in A. FG magnetic beads (1 mg) were incubated with 10 mM berberine derivative berberrubin in N,N-dimethylformamide and 14 mg potassium carbonate at 60°C for 16-20 hours. Unreacted residues were masked with 50% methanol, and the resulting magnetic beads were stored at 4°C.
[0039] Affinity purification with BBR-immobilized magnetic beads:
[0040] With 20mM HEPES-NaOH (pH7.9), 100mM KCl, 1mM MgCl 2 , 0.2mM CaCl 2 , 0.2 mM EDTA, 10% (v / v) glycerol in 100 mM KCl buffer equilibrates BBR immobilized beads (0.5 mg), 0.1% NP-40, 1 mM DTT and 0.2 mM PMSF. Cell extracts were prepared from transfected human 293 cells and rat INS-1 cells as described above and reacted with magnetic beads at 4 °C for 4 h. Wash the beads three times with 100 mM KCl buffer, wash with 1x loading dye solution containing 62.5 mM Tris-HCl (pH 6.8), 0.005% bromophenol blue, 2% SDS, 10% glycerol and 5% ...
Embodiment 1
[0049] Example 1 BBR enhances glucose-stimulated insulin secretion
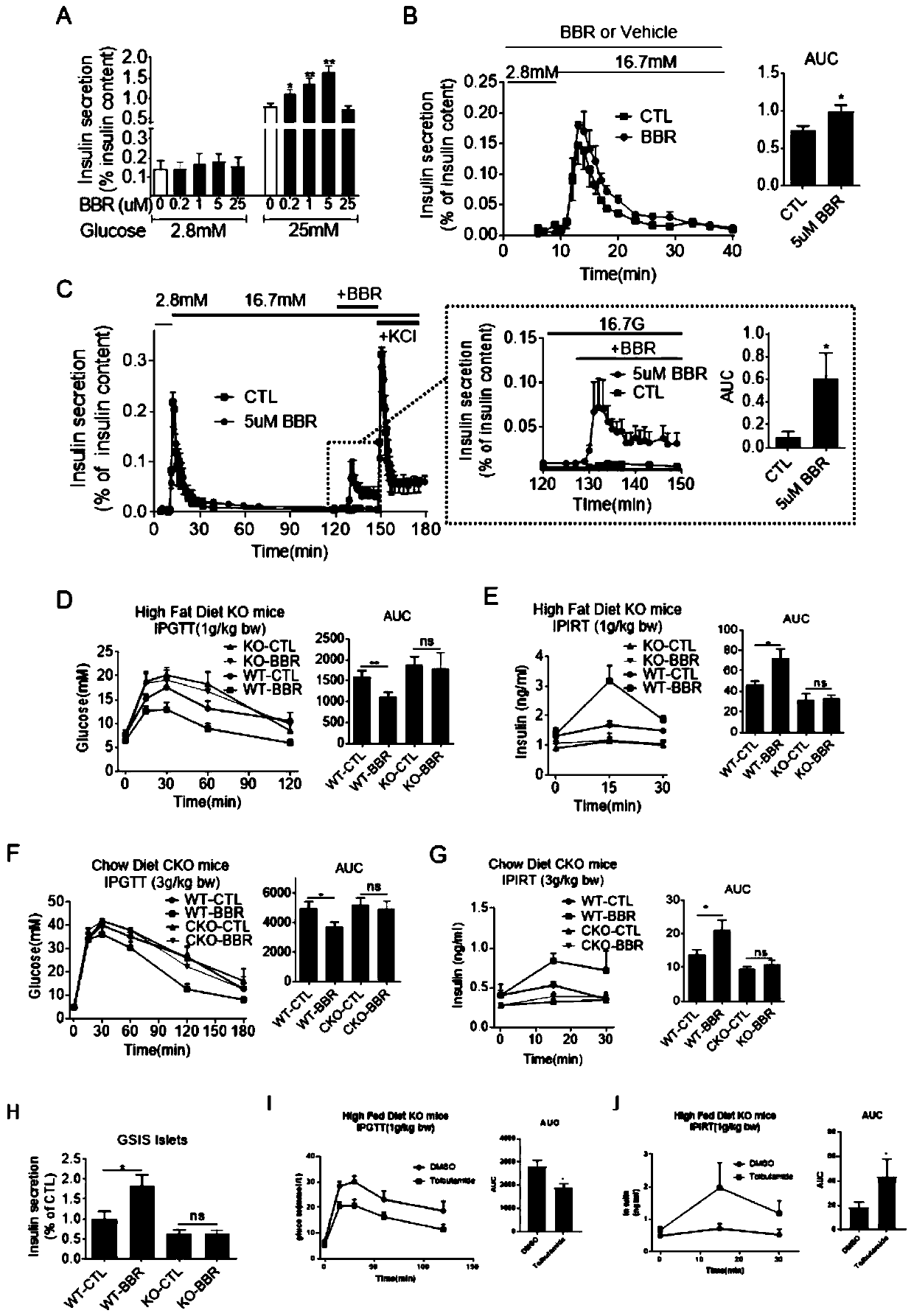
[0050] To examine the role of BBR in insulin secretion, glucose-stimulated insulin secretion (GSIS) experiments were performed in the rat INS-1 pancreatic β-cell line and primary cultured mouse islets. BBR can enhance the glucose-stimulated insulin release of INS-1 cells and islets ( figure 1 A), and in a dose-dependent manner. After adding the drug, the insulin level of the experimental group could be up to 1.7-2.0 times that of the control group. Notably, no insulinotropic effect of BBR was found at basal (low) glucose concentrations ( figure 1 A), which indicates that the insulinotropic effect of BBR is dependent on glucose concentration.
[0051] The effect of BBR on glucose-stimulated insulin secretion was further confirmed by islet perfusion experiments, and islets treated with BBR showed a higher level of insulin secretion compared with the control group ( figure 1 B). Previous studies have shown ...
Embodiment 2
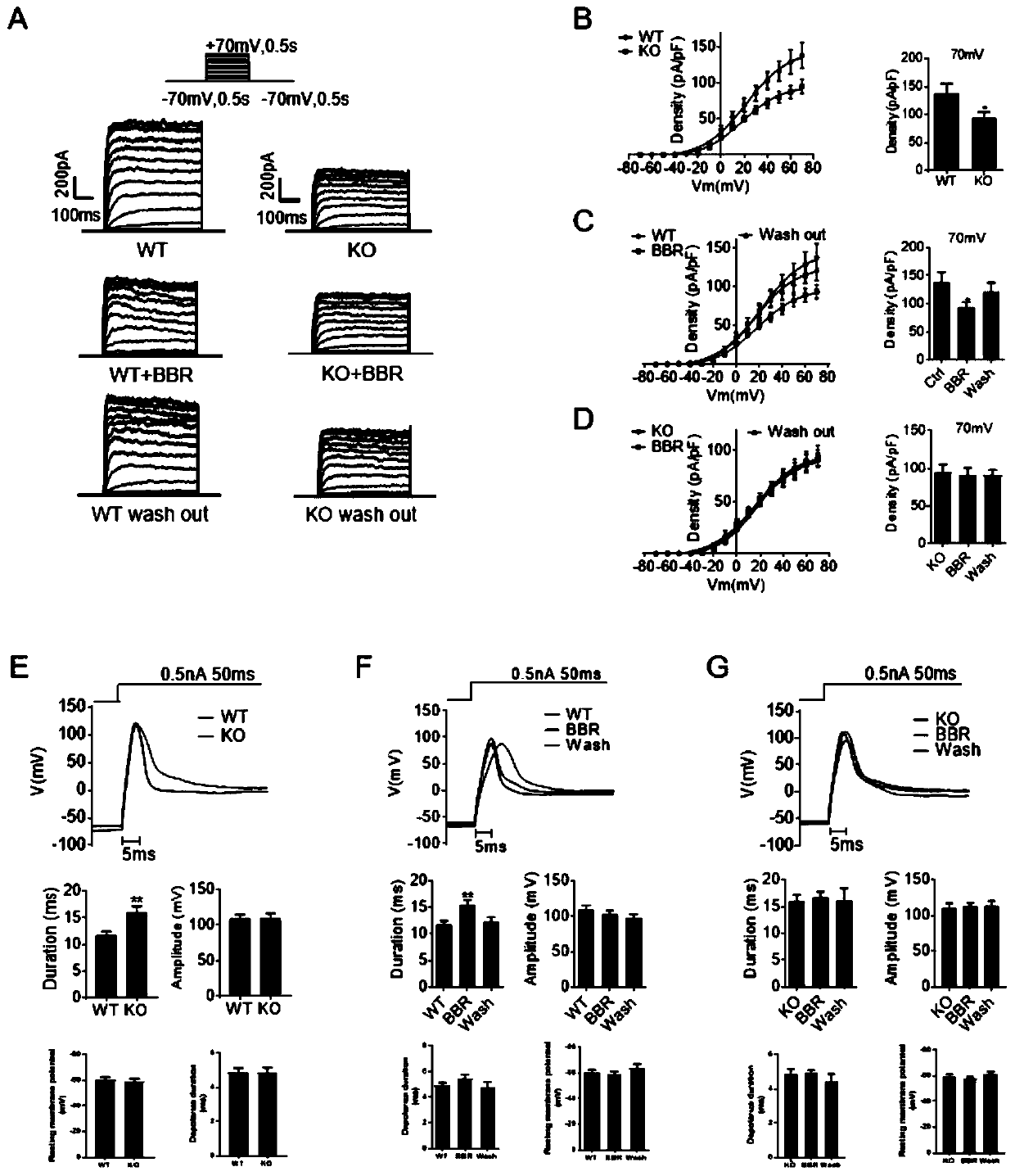
[0054] Example 2 The hypoglycemic effect of BBR in Kcnh6- / - mice is weakened
[0055]The hypoglycemic effect of BBR is more significant in animal models of diabetes, such as: db / db mice, STZ-induced diabetes models and high-fat diet (HFD) models. In order to better verify the hypoglycemic effect of BBR, BBR was applied to an adult animal model—the Kcnh6 gene knockout (KO) mouse model, which exhibited hypoglycemia in childhood and impaired glucose tolerance and even diabetes in adulthood . In the intraperitoneal glucose tolerance test (IPGTT), no obvious hypoglycemic effect of BBR was observed in Kcnh6KO mice. In contrast, WT mice administered BBR showed a marked decrease in blood glucose ( figure 1 D).
[0056] Serum insulin levels during the IPGTT were next measured. KO mice had overall lower insulin levels due to loss of KCNH6 function, whereas BBR failed to increase serum insulin levels in KO mice compared to the significant facilitation observed in WT mice ( figure 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com