Hsd17b13 variants and uses thereof
A DNA targeting and guiding technology, applied in vectors, polymorphic use, nucleic acid vectors, etc., can solve problems such as protective gene variants of chronic liver diseases that have not been described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0456] Example 1. Variant 17β-hydroxysteroid dehydrogenase 13 prevents chronic liver disease
[0457] In the United States, chronic liver disease and cirrhosis are the main causes of higher mortality and morbidity (Kochanek et al., (2016) Natl Vital Stat Rep 65:1-122, the entire content of which is incorporated herein by reference). The most common causes of liver cirrhosis are alcoholic liver disease, chronic hepatitis C and non-alcoholic fatty liver (NAFLD), which results in approximately 80% of patients waiting for liver transplantation (Wong et al., (2015) Gastroenterology 148:547-555 , The entire contents of which are incorporated herein by reference). It is worth noting that in the United States, the prevalence of NAFLD is estimated to reach 19% to 46% (Browning et al., (2004) Hepatology 40:1387-1395; Lazo et al., (2013) Am J Epidemiol 178:38-45 ; And Williams et al., (2011) Gastroenterology 140:124-131, the entire contents of which are incorporated herein by reference) an...
Embodiment 2
[0505] Example 2. rs72613567: The role of TA in HSD17B13 mRNA and HSD17B13 protein expression
[0506] This example examined the effect of the HSD17B13 rs72613567:TA allele on the expression of known and new transcripts of the gene. RNA sequencing was used to evaluate the expression of HSD17B13 mRNA in the histologically normal liver samples of 22 T / T homozygous, 30 T / TA heterozygous and 17 TA / TA homozygous carriers derived from the HSD17B13 rs72613567 splice variant . In addition to the two known HSD17B13 transcripts A and B, a transcript C lacking exon 6 and a transcript D containing an insertion of a guanine nucleotide located at the 3'end of exon 6 were also identified , The insertion of guanine nucleotides can be expected to cause premature truncation of the protein. The transcripts were verified by RT-PCR and Sanger sequencing (data not shown). Long-read cDNA sequencing was also used to verify the D transcript. The expression level of these transcripts varies according ...
Embodiment 3
[0513] Example 3. Variant 17β hydroxysteroid dehydrogenase 13 prevents chronic liver disease
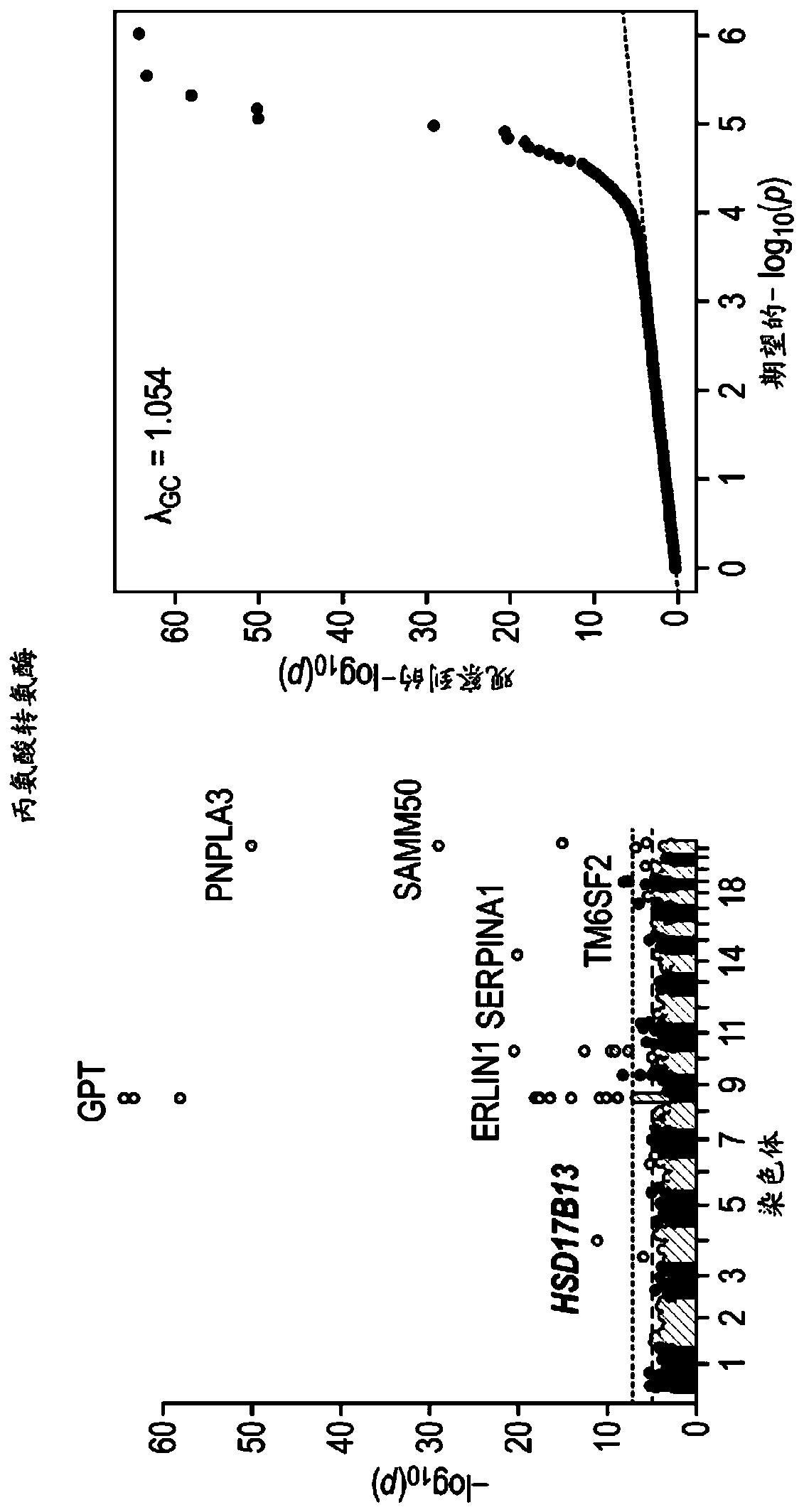
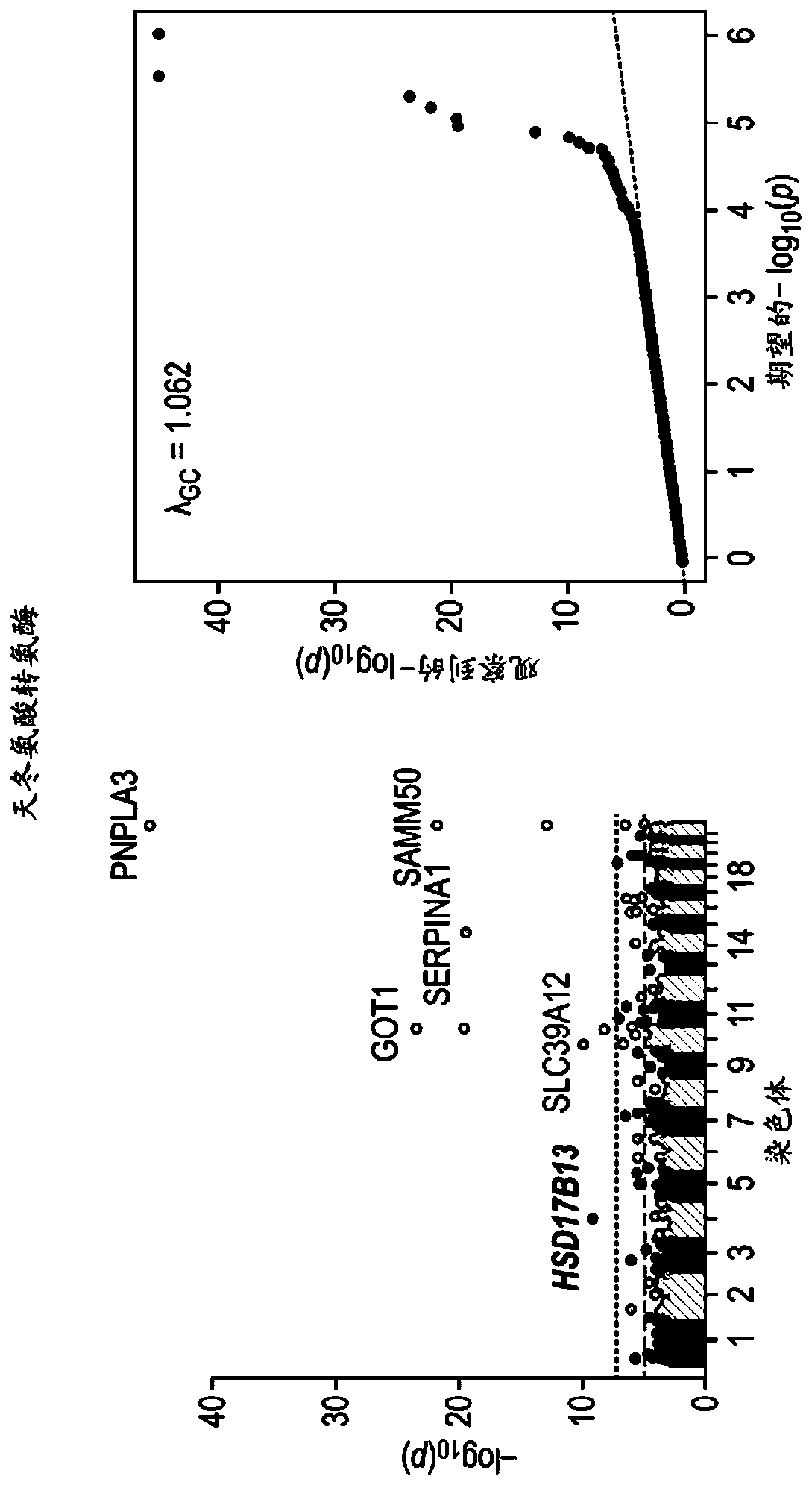
[0514] In order to identify the genetic factors that contribute to chronic liver disease, we used exome sequence data and electronic health records from 46,544 participants in the DiscovEHR human genetic study. We identified gene variants associated with known liver injury biomarkers (serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) to designate candidate variants that may be associated with chronic liver disease. Subsequently, the association of repeated candidate variants in three additional cohorts (12,527 individuals) with the clinical diagnosis of chronic liver disease in DiscovEHR and two independent cohorts (37,892 individuals in total) was evaluated. We also tested the association with the histopathological severity of liver disease in an independent bariatric surgery group (n=2,391 human liver samples).
[0515] The splice variant (rs72613567:TA) in HSD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com