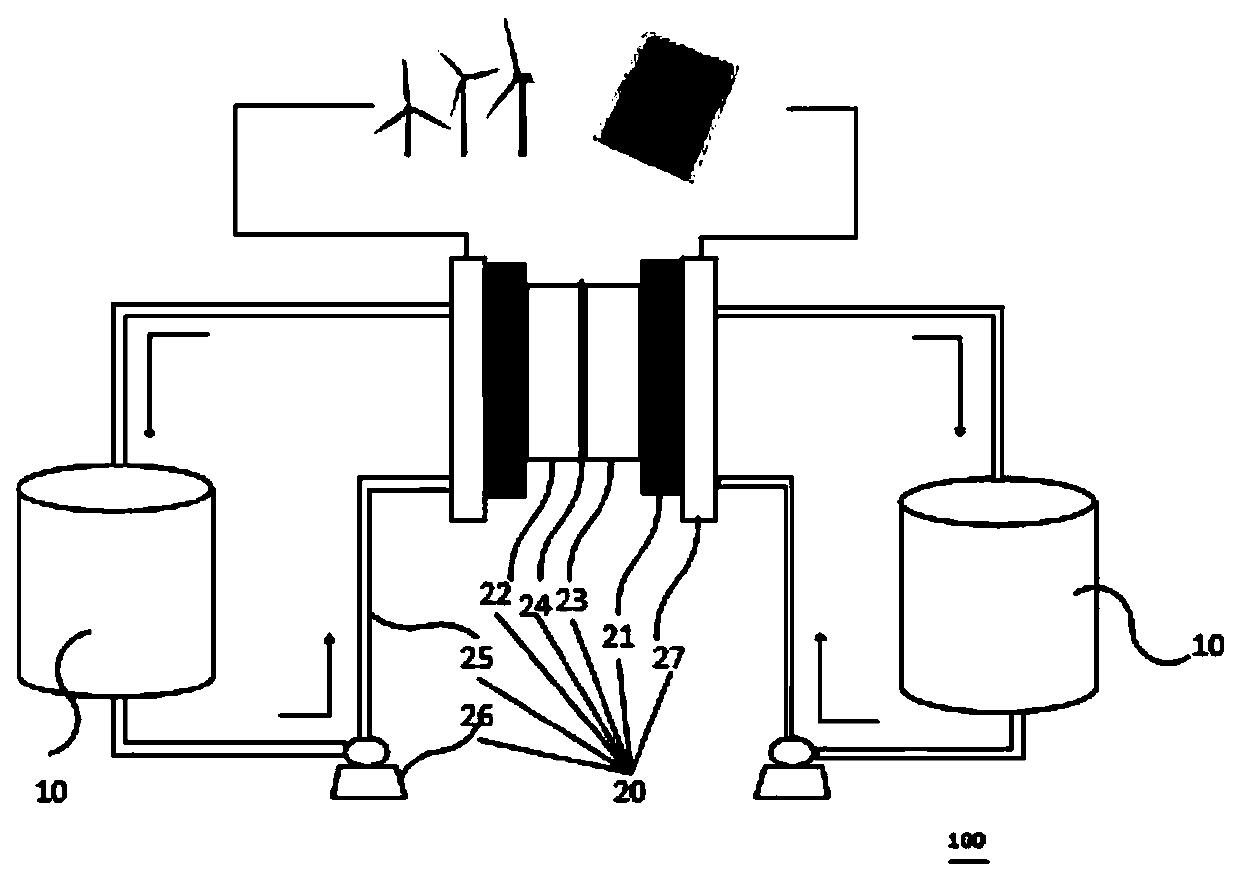
Redox flow battery system based on amino-anthraquinone derivative
An aminoanthraquinone, flow battery technology, applied in the direction of regenerative fuel cells, fuel cells, fuel cell additives, etc., can solve the problems of limited solubility of active materials, prone to water electrolysis side reactions, and prone to cross-contamination of electrolytes, etc. Achieve the effect of low cost, high safety performance and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] It should be noted that the novel anthraquinone derivatives containing carboxyl groups include the following synthetic methods and mainly include the following steps:
[0061] Step 1: Acid Chlorination of Dibasic Acids Containing Carboxyl Terminals
[0062] Put the carboxyl-terminated dibasic acid and thionyl chloride into the reactor, then add toluene as the reaction solvent, add an appropriate amount of catalyst for catalysis, heat up to 60°C for reaction, and distill off the solvent and chlorination after the reaction is over. Sulfoxide was added to toluene for distillation (20mL×2), and the residue was used for further reaction. The reaction process is as follows:
[0063]
[0064] The second step: the synthesis of aminoanthraquinone containing carboxyl group
[0065]Mix the product obtained in the first step with aminoanthraquinone and put it into the reactor, then add toluene as the reaction solvent, heat up to reflux reaction, and distill off the solvent und...
Embodiment 1
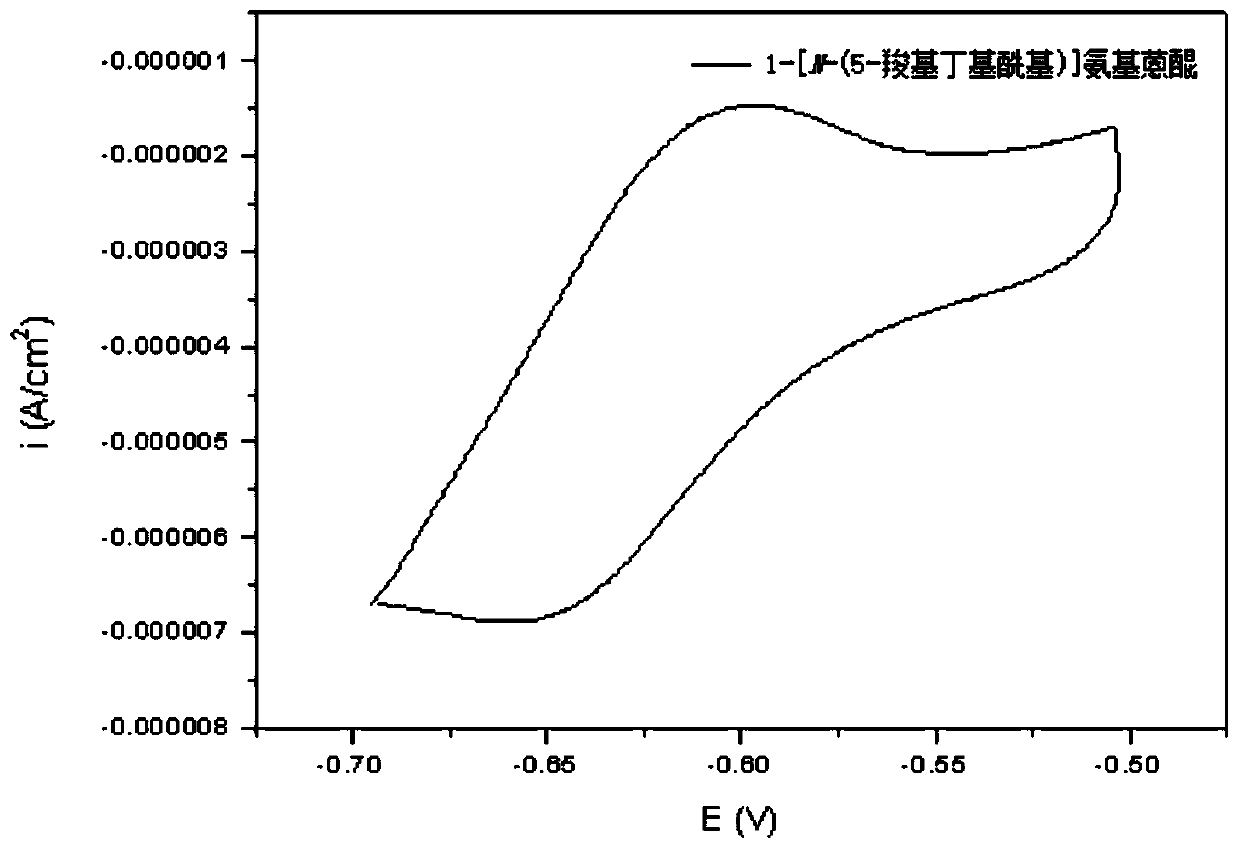
[0089] Synthesis of 1-[N-(6-carboxypentyl)]aminoanthraquinone
[0090] 2.92g of adipic acid (0.02mol) and 15mL of thionyl chloride were mixed and dissolved in 35mL of toluene, and 0.01g of DMF was added as a catalyst. The temperature was raised to 60°C and the reaction was refluxed, and the reaction was stopped when the solvent was light yellow (12h-24h). Thionyl chloride and toluene were distilled off under reduced pressure, and toluene was added for distillation (20 mL×2), and the residue was used for the following reaction.
[0091] Add 40 mL of toluene and 0.89 g of 1-aminoanthraquinone to the above residue in sequence, and slowly raise the temperature to reflux. As the reaction progressed, the reaction solution gradually changed from red to orange-yellow. The progress of the reaction was monitored by TLC and the reaction was stopped when the reaction was almost complete (15h-20h). The solvent toluene was distilled off under reduced pressure (to be completely steamed as...
Embodiment 2
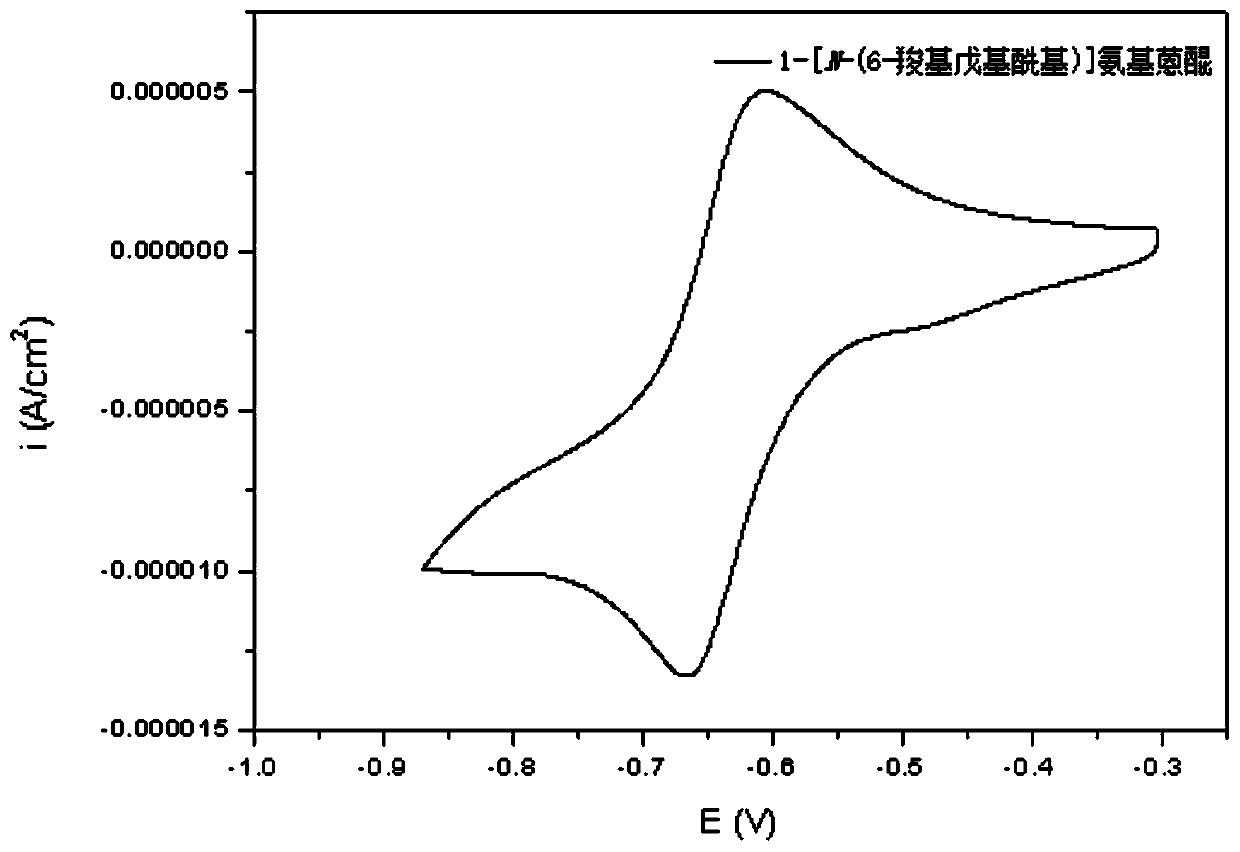
[0093] Synthesis of 1-[N-(8-carboxyheptyl)]aminoanthraquinone
[0094] 3.48g of suberic acid (0.02mol) and 15mL of thionyl chloride were mixed and dissolved in 35mL of toluene, and 0.01g of pyridine was added as a catalyst. The temperature was raised to 60°C and the reaction was refluxed, and the reaction was stopped when the solvent was light yellow (12h-24h). Thionyl chloride and toluene were distilled off under reduced pressure, and toluene was added for distillation (20 mL×2), and the residue was used for the following reaction.
[0095] Add 40 mL of toluene and 0.89 g of 1-aminoanthraquinone to the above residue in sequence, and slowly raise the temperature to reflux. As the reaction progressed, the reaction solution gradually changed from red to orange-yellow. The progress of the reaction was monitored by TLC and the reaction was stopped when the reaction was almost complete (15h-20h). The solvent toluene was distilled off under reduced pressure (to be steamed out com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com