Application of mesenchymal stem cell paracrine factor in eye drops
A kind of stem cell and paracrine technology, applied in the field of biological stem cell technology in the field of ophthalmology, can solve the problems of non-discovery, permanent blindness, difficulty, etc., and achieve the effects of improving vision, reducing local inflammatory response and degeneration, and being easy to prepare.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
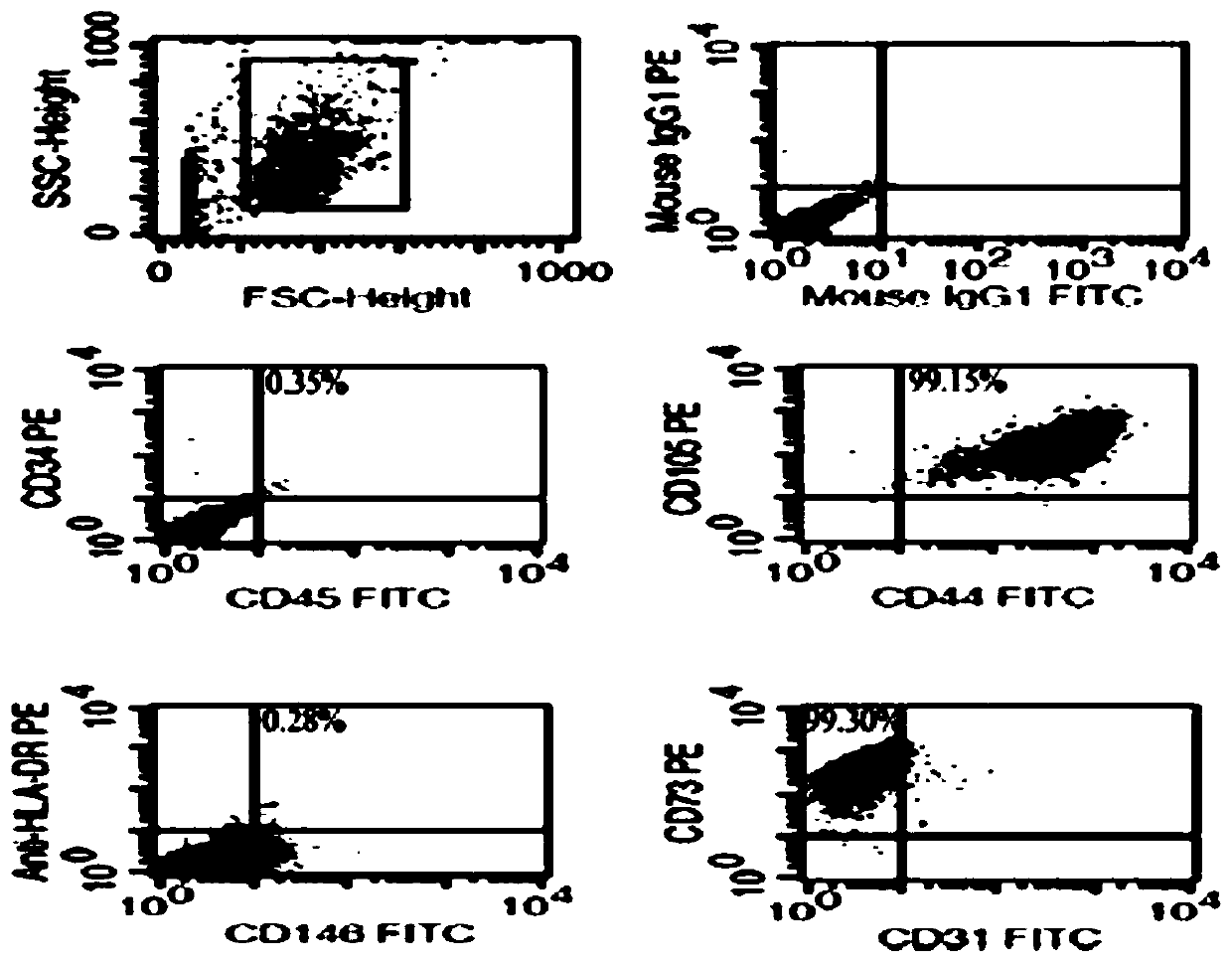
[0049] Example 1 Preparation of Umbilical Cord Mesenchymal Stem Cell Paracrine Factor Eye Drops
[0050] The umbilical cord mesenchymal stem cell eye drops of the present invention are mainly derived from human umbilical cord mesenchymal stem cells, but the source of stem cells is not limited to the umbilical cord, and can be derived from multiple human tissue sources such as human placenta, bone marrow, dental pulp, and fat.
[0051] Wherein, the preparation process of umbilical cord mesenchymal stem cell paracrine factor eye drops is as follows:
[0052] (1) Cultivation of umbilical cord mesenchymal stem cells: cut fresh umbilical cord, wash it with sterile saline, further remove visible blood vessels, cut the umbilical cord to a small tissue piece about 2-3cm in size, and put it into a cell culture dish Middle, cut the umbilical cord Wharton's jelly: cut the washed umbilical cord Wharton's jelly into 1-3mm tissue fragments, and centrifuge; remove the supernatant from the ce...
Embodiment 2
[0055] Example 2 Ocular Safety Evaluation Test of Mesenchymal Stem Cell Paracrine Factor Eye Drops:
[0056] Randomly select 24 healthy SD rats of any sex and divide them into two groups: one group is used as blank control, the left eye is not treated, and the right eye is given medical saline; the other group is used as the experimental group, the left eye is not treated, and the right eye is given this Invented umbilical cord mesenchymal stem cell paracrine factor eye drops. 3 times a day, 1-2 drops (about 200μl / drop) at a time, continuous eye drops for 14 days. During this period, two time points of 7 days and 14 days were selected to observe the physiological changes of the rats. According to the Draize scoring standard, the performance of rat eyes was scored for irritation. No irritation is 0-3 points; mild stimulation is 4-8 points; moderate stimulation is 9-12 points; severe stimulation is 13-16 points. The final results showed that instillation of normal saline and ...
Embodiment 3
[0057] Example 3 Human eye safety evaluation test of mesenchymal stem cell paracrine factor eye drops:
[0058] Randomly select 30 volunteers (regardless of gender) with single-eye point umbilical cord mesenchymal stem cell paracrine factor eye drops. 3 times a day, 1-2 drops (about 300μl / drop) at a time, continuous eye drops for 14 days. During the period, two time points of 7 days and 14 days were selected for visual acuity test, fundus test, blood routine test and biochemical test. The final results showed that the volunteers did not see eye redness and swelling, increased eye secretions or any other discomfort at the two time points of 7 days and 14 days after dripping mesenchymal stem cell exosome eye drops. At the same time, no abnormalities were found in the fundus examination , Blood routine and biochemical tests were normal. After three months and half a year, 30 volunteers were re-examined, and all symptoms and blood indicators were normal. The above fully shows t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com