Method to distinguish between peroxidase-activated proliferator receptor gamma full agonist, partial agonist, and antagonist activity
A peroxidase and agonist technology, applied in the field of endocrine disruptor identification, can solve the problems of low sampling efficiency, discrimination of PPARγ interfering substances, inability to distinguish activities of complete agonists, partial agonists, and antagonists, and save the laboratory Cost and effect of workload reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
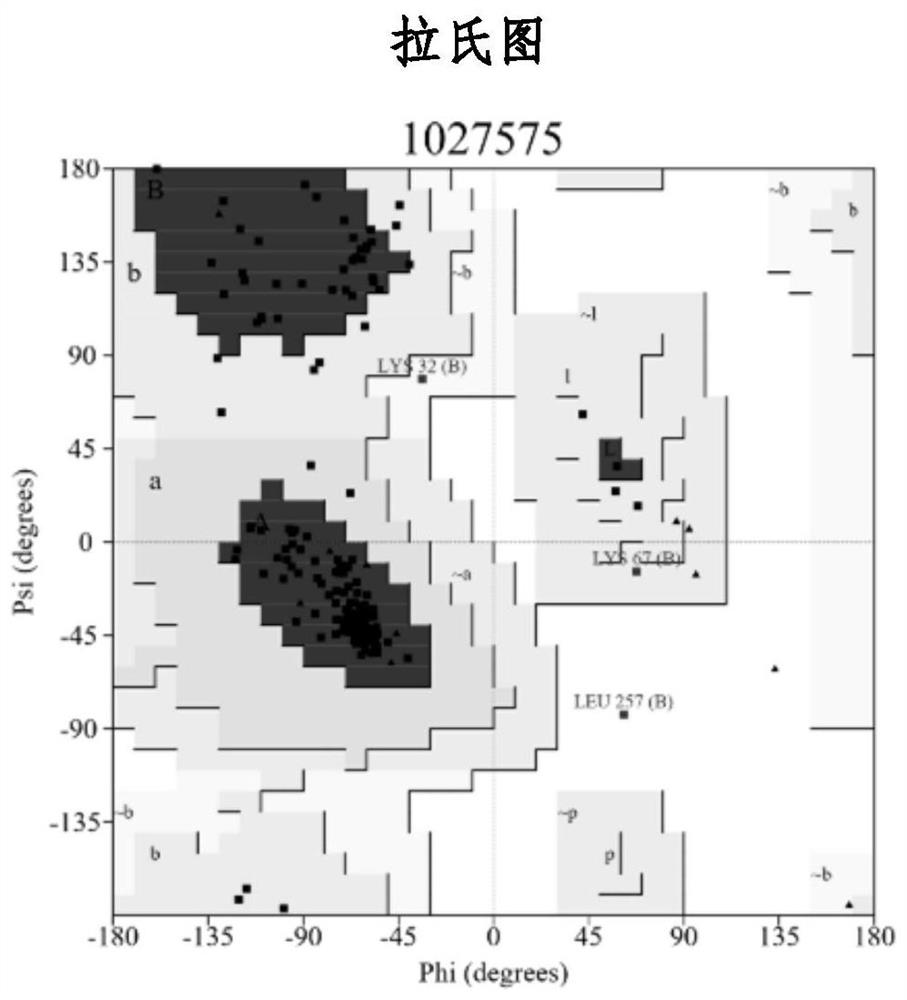
[0036] Research material and data collection of the present invention:
[0037] The substance used in this study is the substance that Mingliang Fang et al. analyzed from background indoor dust in 2015. The researcher analyzed a variety of substances from indoor dust in Beijing, including a variety of flame retardants and their metabolites. From PPARγ activation and binding experiments, it can be found that most of these substances have been confirmed as full agonists or partial agonists of PPARγ in previous studies. The researcher used rosiglitazone and the endogenous ligand 15d-PJG2 of PPAR as positive substances, and used the reporter gene method to study the PPARγ activity of these substances. Activity data for this study were derived from this study. Before using the data, the EC15 for each substance was first converted to equivalents relative to rosiglitazone and then logarithmic.
[0038] Receptor and Ligand Modeling:
[0039] The protein molecular structure templat...
Embodiment 2
[0051] Embodiment 1 experimental result:
[0052] The substance used in this study is the substance that Mingliang Fang et al. analyzed from background indoor dust in 2015. PPARγ activity data and affinity data for PPARγ binding ligands are derived from this researcher's article. The results are shown in Table 1.
[0053] Table 1 Substances and their activities in the literature NA: No definite data
[0054] compound EC15(μM) TPP 2.12 TPPi NA mono-ITP 3.6 Di-ITP 3.25 Tri-ITP 5.7 BYZGR 5.86 2,4,6-TIP 8.72 2,4,6-TBP 5.89 TCBPA 0.23 TBBPA 0.32 TBPP NA TBOEP NA TBBA 8.16 BDE47 5.2 3-OH-BDE47 2.01 6-OH-BDE47 NA TCS NA DiBP 4.47 DBP 6.73 BzB 2.94 TBMEHP 0.53 MEHP 1.26 15d-PJG2 0.51 rosiglitazone (positive control) 0.00132
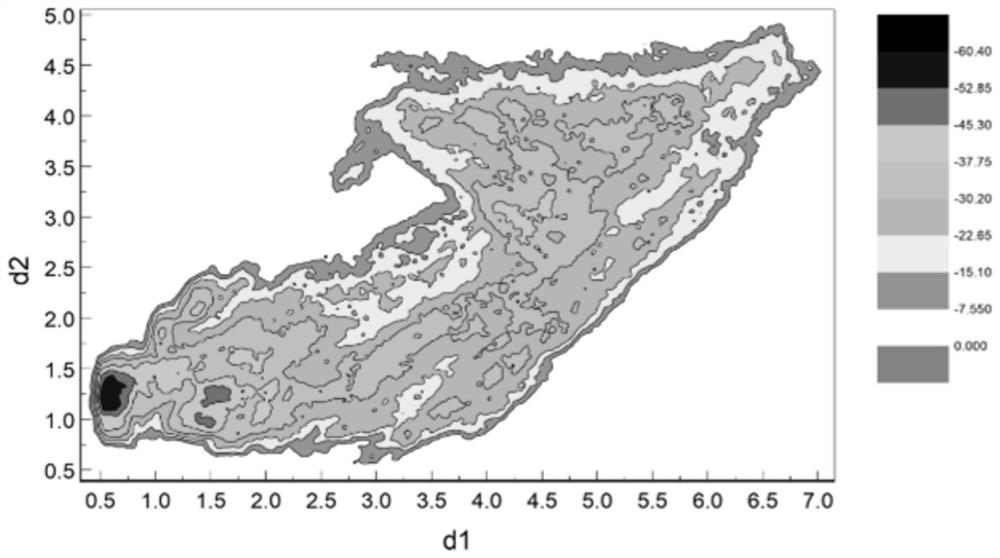
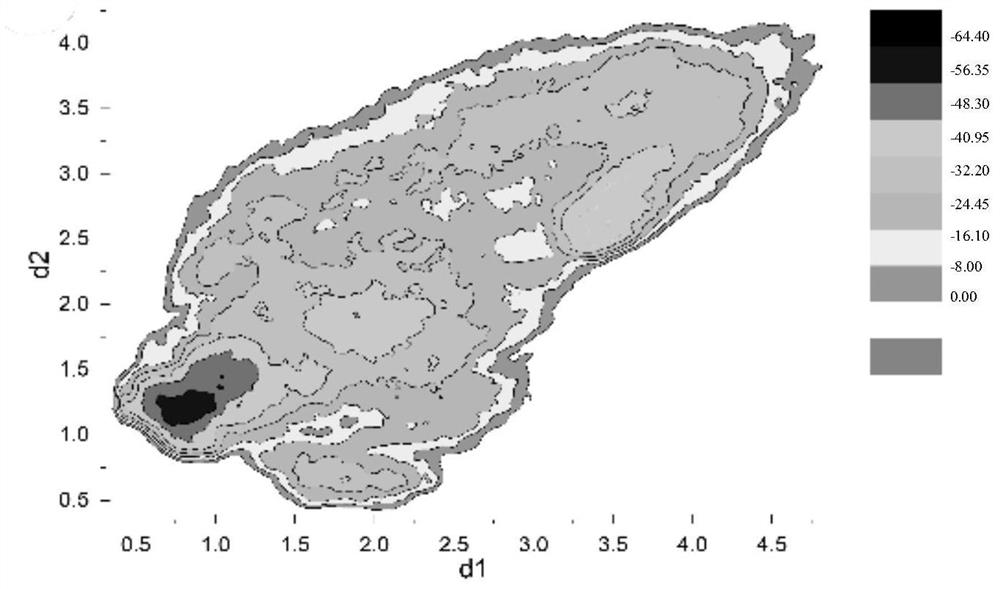
[0055] H12 conformation and activity differentiation of PPARγ:
[0056] The free energy spectra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com