Roxatidine acetate hydrochloride slow-release capsules and preparation method thereof
A technology of roxatidine hydrochloride acetate and sustained-release capsules is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, and can solve problems such as no domestic and foreign literature reports, etc., Achieve the effect of improving medication compliance, less coating loss, and reducing the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
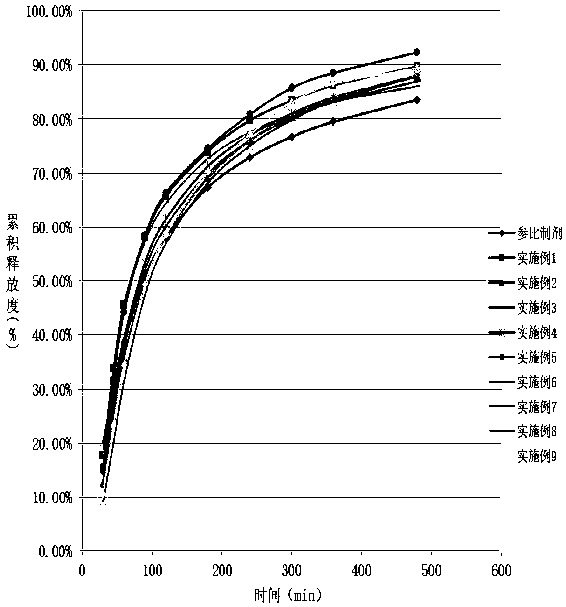
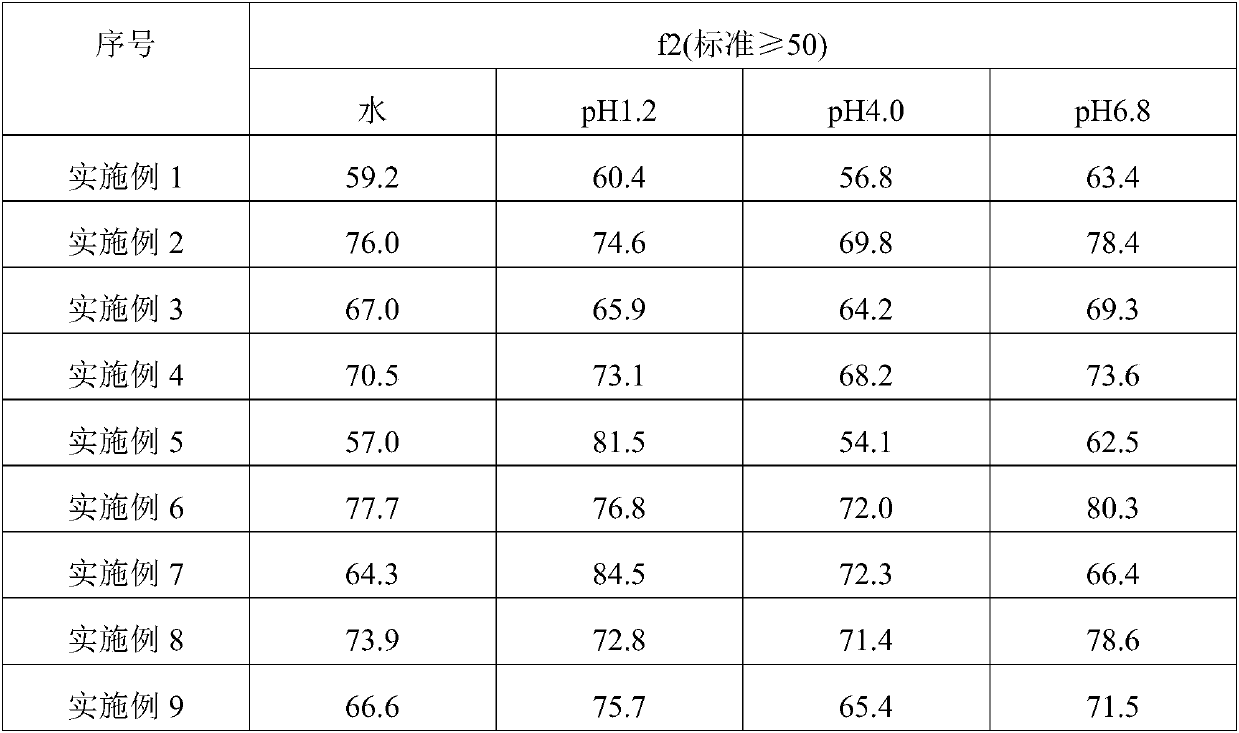
Image
Examples
Embodiment 1
[0039] 1), Preparation of drug-loaded pellets:
[0040] (1) Weigh 600.0 g of purified water and add it to the liquid mixing tank, start stirring, add 750.0 g of roxatidine hydrochloride acetate, and stir to dissolve.
[0041] (2), take 675.0g of sucrose ball core, place in the bottom spray fluidized bed, set material temperature 50 ℃ to carry out drug-containing layer pellet coating, coating time 2 hours;
[0042] (3), after coating, dry to moisture<0.5%;
[0043] (4), sieving the dried ball core to obtain drug-loaded pellets;
[0044] 2), preparation of sustained-release pellets:
[0045] (1) Weigh 750.0g of ethanol and 300.0g of purified water, add them to the liquid mixing tank, start stirring, add 75.0g of ethyl cellulose, stir until dissolved, then add 7.5g of triethyl citrate and colloidal Silicon dioxide 7.5g, stir well;
[0046] (2) Place the drug-loaded pellets in the bottom spray fluidized bed, set the temperature of the material at 30°C to coat the pellets with ...
Embodiment 2
[0052] 1), Preparation of drug-loaded pellets:
[0053] (1) Weigh 750.0 g of purified water and add it to the liquid mixing tank, start stirring, add 750.0 g of roxatidine hydrochloride acetate, and stir to dissolve;
[0054] (2), take 712.5g of sucrose ball core, place in the bottom spray fluidized bed, set material temperature 55 ℃ to carry out drug-containing layer pellet coating, coating time 3 hours;
[0055] (3), after coating, dry to moisture<0.5%;
[0056] (4), sieving the dried ball core to obtain drug-loaded pellets;
[0057] 2), preparation of sustained-release pellets:
[0058] (1) Weigh 1125.0g of ethanol and 450.0g of purified water, add them to the liquid mixing tank, start stirring, add 112.5g of ethyl cellulose, stir until dissolved, then add 11.2g of triethyl citrate and colloidal Silicon dioxide 11.2g, stir well;
[0059] (2) Place the drug-loaded pellets in the bottom spray fluidized bed, set the temperature of the material at 35°C to coat the pellets w...
Embodiment 3
[0065] 1), Preparation of drug-loaded pellets:
[0066] (1) Weigh 900.0 g of purified water and add it to the liquid mixing tank, start stirring, add 750.0 g of roxatidine hydrochloride acetate, and stir to dissolve;
[0067] (2), take 750.0g of sucrose ball core, place in the bottom spray fluidized bed, set material temperature 60 ℃ to carry out drug-containing layer pellet coating, coating time 4 hours;
[0068] (3), after coating, dry to moisture<0.5%;
[0069] (4), sieving the dried ball core to obtain drug-loaded pellets;
[0070] 2), preparation of sustained-release pellets:
[0071] (1) Weigh 1500.0g of ethanol and 600.0g of purified water, add them to the liquid mixing tank, start stirring, add 150.0g of ethyl cellulose, stir until dissolved, then add 15.0g of triethyl citrate and colloidal Silicon dioxide 15.0g, stir well;
[0072] (2) Place the drug-loaded pellets in the bottom spray fluidized bed, set the material temperature to 40°C to coat the sustained-release ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com