Process for preparing (9e,11z)-9, 11-hexadecadienal
A technology of carbadienal and 11Z, which is applied in the field of preparation of -9,11-hexadecadienal, which can solve problems such as outbreaks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
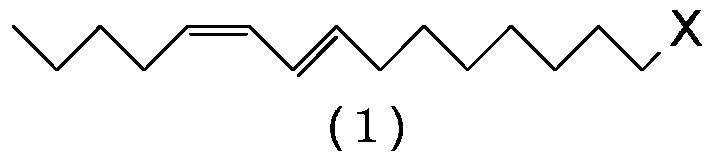
[0100] Preparation of (8E,10Z)-1-chloro-8,10-pentadecadiene (1:X=Cl)
[0101] Magnesium (17.8 g, 0.733 gram atom) and tetrahydrofuran (198 g) were added to the reactor, and the resulting mixture was stirred at 60˜65° C. for 30 minutes. Then, (4E,6Z)-1-chloro-4,6-undecadadiene (5:X=Cl) (124g, 0.667mol) was added dropwise at 60-70°C, and the resulting mixture was heated at 70-75°C Stir for 6 hours to prepare (4E,6Z)-4,6-undecadienylmagnesium chloride. In another reactor was charged cuprous iodide (1.27 g, 0.00667 mol), triethyl phosphite (2.66 g, 0.0160 mol), 1-bromo-4-chlorobutane (131 g, 0.767 mol) and tetrahydrofuran ( 66.1 g), and the resulting mixture was stirred at 0-5°C for 30 minutes. Then, the solution of (4E,6Z)-4,6-undecadienylmagnesium chloride prepared above in tetrahydrofuran was added dropwise at 5˜15°C. After the dropwise addition was completed, the resulting reaction mixture was stirred at 5˜10° C. for 2 hours, and then the reaction was terminated by adding...
Embodiment 2
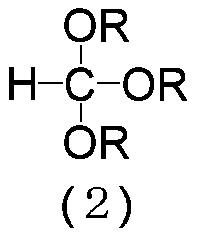
[0107] (9E,11Z)-1,1-diethoxy-9,11-hexadecadiene (3: R=C 2 h 5 ) preparation
[0108]Magnesium (7.82 g, 0.322 gram atom) and tetrahydrofuran (87.8 g) were added to the reactor, and the resulting mixture was stirred at 60˜65° C. for 30 minutes. Then, (8E,10Z)-1-chloro-8,10-pentadecadiene (1:X=Cl) (71.1g, 0.293mol) was added dropwise at 60~70℃, and then the resulting mixture was heated at 70~ Stir at 75°C for 2 hours to prepare (8E,10Z)-8,10-pentadecadienylmagnesium chloride. Then, toluene (136 g) and triethyl orthoformate (56.4 g, 0.380 mol) were added to the reactor at 75˜85° C., and the resulting mixture was stirred at 90˜100° C. for 17 hours. It was cooled to 0˜10° C., and then 20% by weight aqueous hydrogen chloride solution (40.5 g), water (43.9 g) and acetic acid (8.08 g) were added to the reaction mixture. After removing the aqueous layer from the resulting reaction mixture by liquid-liquid separation, the organic layer was washed with an aqueous solution of 8% by ...
Embodiment 3
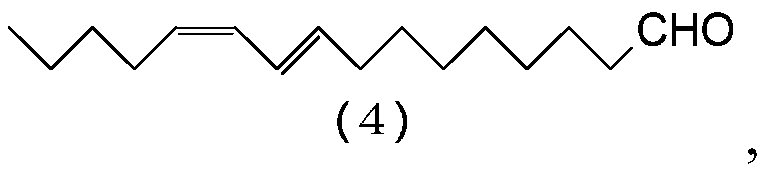
[0114] Preparation of (9E,11Z)-9,11-Hexadecadienal (4)
[0115] Add (9E,11Z)-1,1-diethoxy-9,11-hexadecadiene (3:R=C 2 h 5 ) (146g, 0.469mol), tetrahydrofuran (469g), oxalic acid dihydrate (177g, 1.41mol) and water (469g), and the resulting mixture was stirred at 60-65°C for 3 hours. It was cooled to 40˜50° C., and hexane (138 g) was added to the mixture. After removing the aqueous layer from the resulting reaction mixture by liquid-liquid separation, the organic layer was washed with a solution of sodium chloride (4.27 g) and water (286 g). The organic layer was concentrated by evaporating the solvent in vacuo, and the resulting concentrate was distilled in vacuo to give (9E,11Z)-9,11-hexadecadienal (4) (93.0 g, 0.394 mol) in a yield of 83.9 %.
[0116] Characterization of (9E,11Z)-9,11-Hexadecadienal (4)
[0117] [NMR spectrum] 1 H-NMR (500MHz, CDCl 3 ):δ0.90(3H,t,J=6.9Hz),1.29-1.41(12H,m),1.62(2H,tt,J=7.3,7.3Hz),2.08(2H,dt,J=6.9,6.9 Hz), 2.15(2H, dt, J=7.3, 7.3Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com