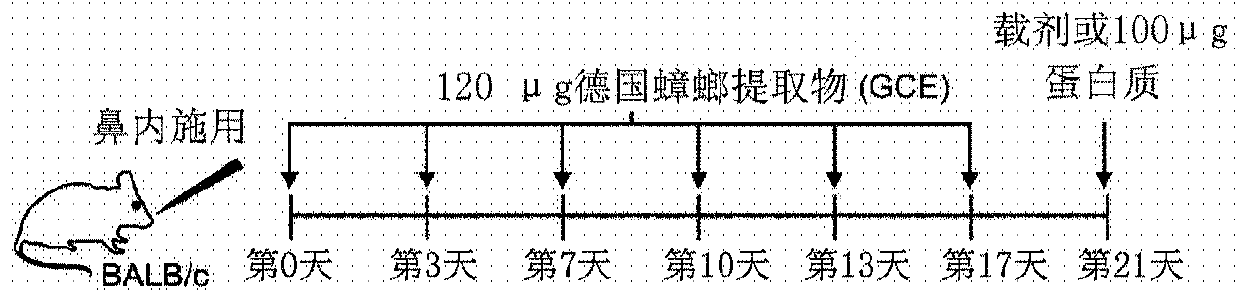
Pharmaceutical composition for prevention or treatment of inflammatory respiratory disease, with fusion protein of cell permeable peptide and ctctla4 peptide contained as effective ingredient therein
A technology for respiratory diseases and fusion proteins, applied in the field of pharmaceutical compositions for the prevention or treatment of inflammatory respiratory diseases comprising a fusion protein in which a cell-penetrating peptide is fused with a CTCTLA4 peptide as an active ingredient, capable of solving topical administration Difficulties, side effects, susceptibility to infection, etc., to achieve the effect of reducing airway hyperresponsiveness, inhibiting airway inflammation, rapid and effective treatment or prevention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0091] Synthesis and purification of dNP2, ctCTLA-4 and Hph-1 peptides
[0092] Peptides having the amino acid sequences of SEQ ID NOs 1 to 3 were synthesized.
[0093] The cell penetrating peptide having the amino acid sequence of SEQ ID NO 1 was named 'dNP2', the peptide having the amino acid sequence of SEQ ID NO 2 was named 'ctCTLA-4' and the cell penetrating peptide having the amino acid sequence of SEQ ID NO 3 The transpeptide was named 'Hph-1'.
[0094] After synthesis of the sense and antisense oligodeoxynucleotides of the amino acid sequence, they were kept at 95° C. for 3 minutes to remove any secondary or tertiary structures formed (denaturation). Then, double-stranded DNA was prepared by changing the temperature to 50°C and then to 72°C. For insertion into the pRSET-b vector, in addition to sense and antisense oligodeoxynucleotides, restriction enzyme specific sequences were inserted at the 5' and 3' ends. Then, Escherichia coli BL21(DE3)Star pLysS was transfor...
Embodiment 1
[0101] Preparation of dNP2-ctCTLA-4 fusion protein
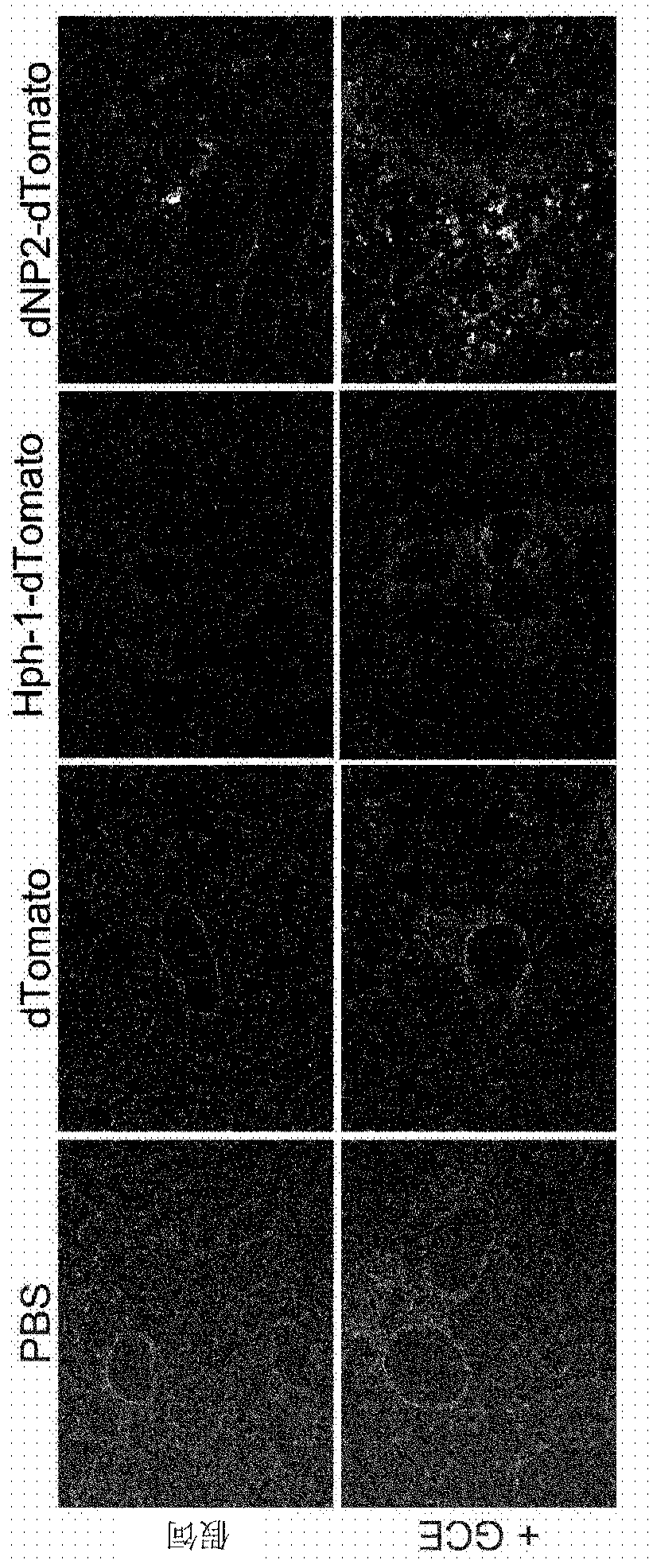
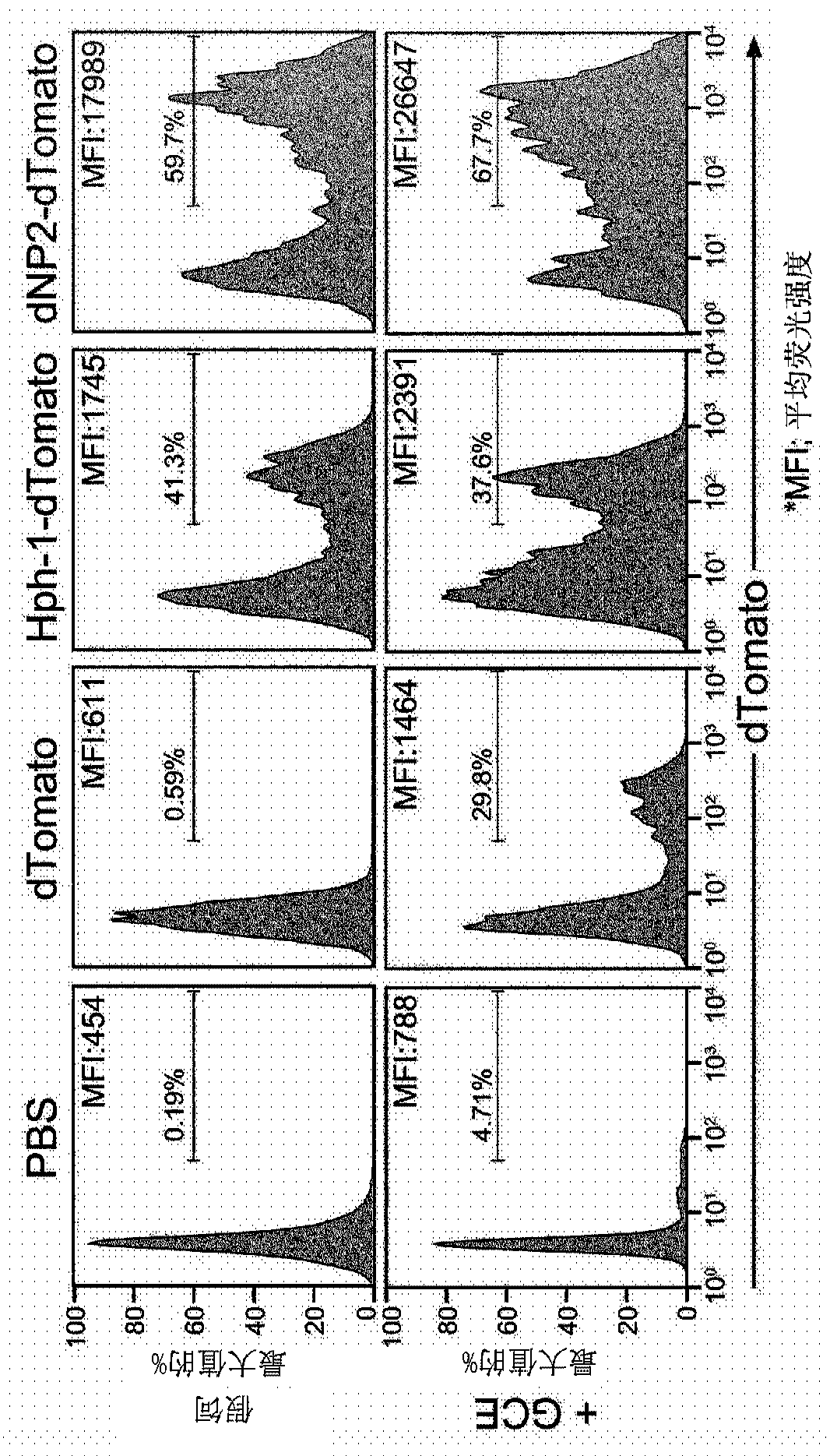
[0102] In order to fuse the cell penetrating peptide having the amino acid sequence of SEQ ID NO 1 prepared in Preparation Example 1 with the ctCTLA-4 peptide having the amino acid sequence of SEQ ID NO 2, a peptide that allows the cell penetrating peptide represented by SEQ ID NO 1 was prepared. Primer linked to the N-terminus of the ctCTLA-4 peptide. After generating the dNP2-ctCTLA-4 gene by PCR reaction, it was inserted into the vector (pRSET-b). After purification of proteins expressed in E. coli, intracellular delivery efficiency was investigated. Detailed experimental procedures are described below.
[0103] 1) Preparation of coding gene
[0104] By inserting the DNA base sequence encoding the cell penetrating peptide having the amino acid sequence of SEQ ID NO 1 prepared in Preparation Example 1 into a part of the N-terminus encoding the ctCTLA-4 peptide having the amino acid sequence of SEQ ID NO 2 The forward ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com