Immunosuppressive polypeptide designed by acidic amino acid scanning method and application thereof
An amino acid, autoimmune technology, applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Separation and purification of sequence recombinant polypeptides with different distribution of aspartic acid residues
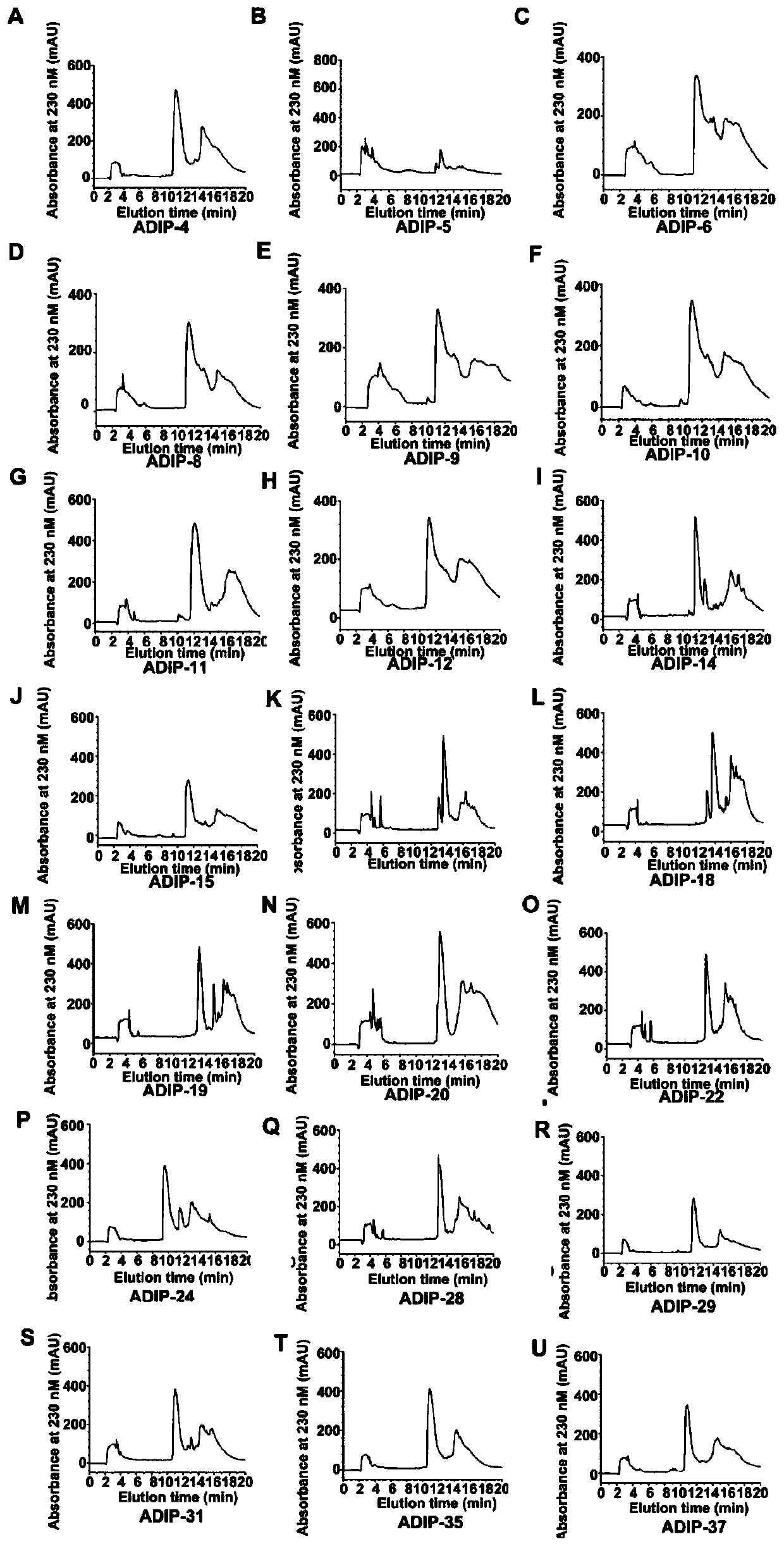
[0060] The present invention adopts the genetic engineering technology (ZL200710053679.0; ZL201310208475.5; Structural Basis of a Potent Peptide Inhibitor Designed for Kv1.3Channel, a Therapeutic Target of AutoimmuneDisease. J Biol Chem,2008,283:19058-19065;Unusual binding mode of scorpiontoxin BmKTX onto potassium channels relies on its distribution of acidic residues.Biochem Biophys Res Commun,2014,447(1):70-76;Toxin design of acid residue-evolutioned fudition de novo peptide drugs forimmunotherapeutic target Kv1.3channel. Scientific Reports, 2015, 5:9881) to prepare a series of peptides, and separate recombinant peptides by reversed-phase high performance liquid chromatography. SEQ ID NO: 1 ~ 21 peptide separation chromatogram as shown in figure 1 shown.
Embodiment 2
[0061] Example 2: Identification of sequence recombinant polypeptides with different distribution of aspartic acid residues
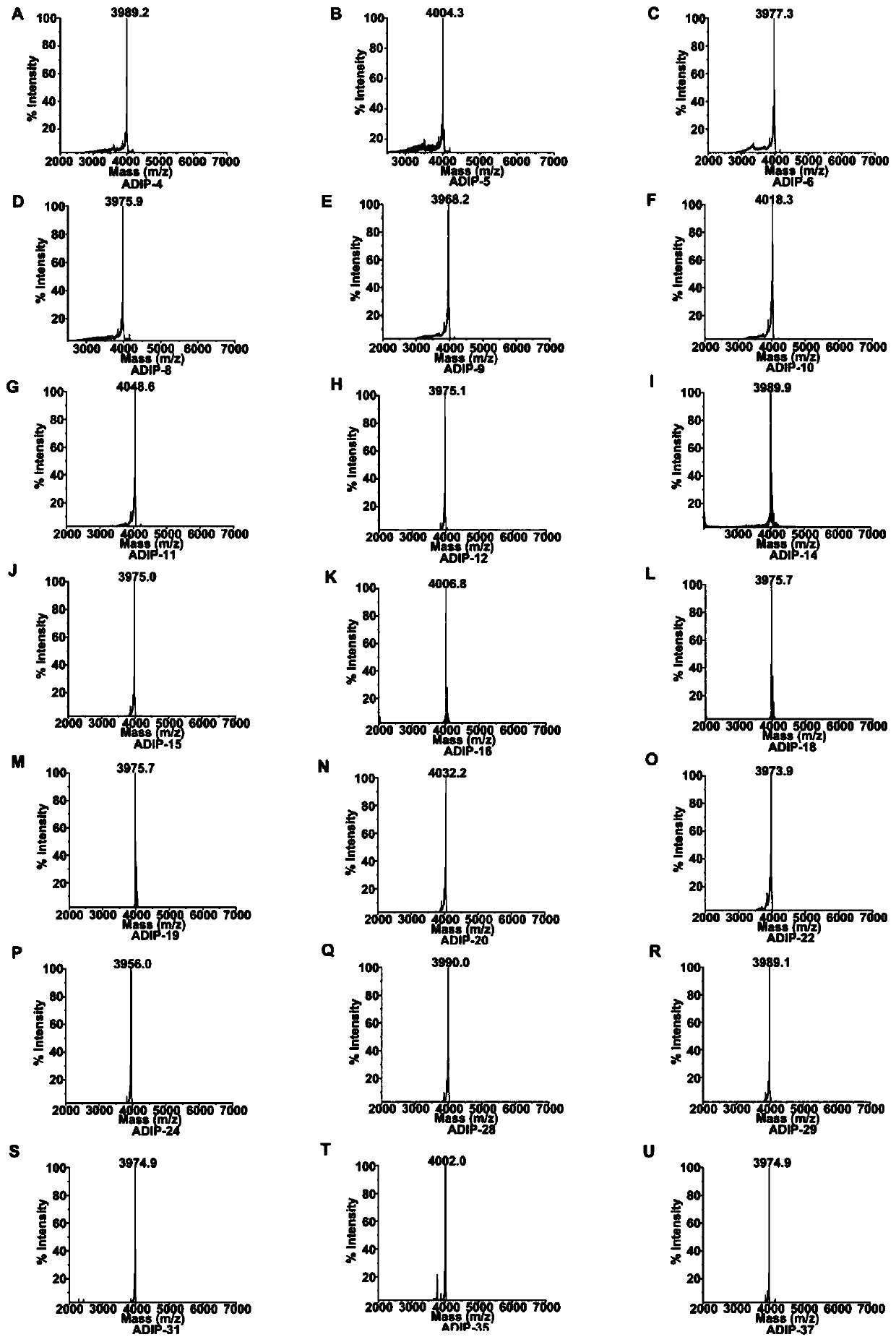
[0062] Using mass spectrometry to perform mass spectrometry analysis on the polypeptide provided by the present invention with the amino acid sequence shown in SEQ ID NO: 1-21, the results are as follows figure 2 shown. After mass spectrometry analysis, the determined molecular weight of these peptides is consistent with the theoretical molecular weight deduced from the amino acid sequence.
Embodiment 3
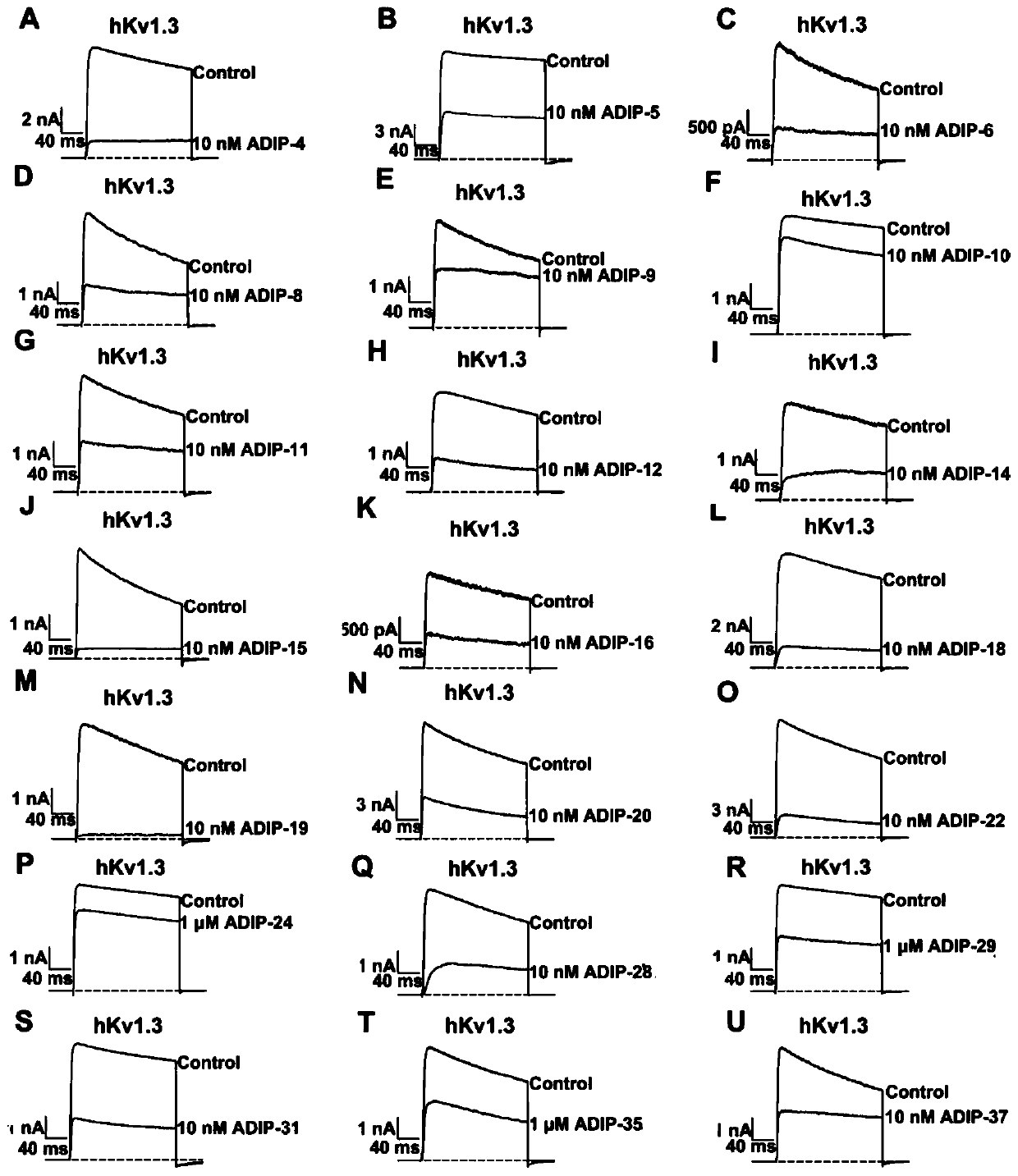
[0063] Example 3: Analysis of the pharmacological activity of recombinant polypeptides with different distributions of aspartic acid residues on the potassium channel Kv1.3
[0064] HEK-293T cells were cultured in DMEM medium containing 10% fetal bovine serum at 37°C, 5% CO 2 Conditioned culture, the potassium channel Kv1.3 recombinant plasmid was used Sofast TM The transfection kit was used for transfection, and the transfected cells were selectively cultured on 0.8 mg / mL Geneticin medium. The pharmacological activity of the polypeptide having the amino acid sequence shown in SEQ ID NO: 1-21 is determined and analyzed by using the instrument for whole-cell patch clamp (EPC-10 dual-channel patch clamp amplifier HEKA, Elektronik, Lambrecht, Germany). analyze. The setting of experimental parameters, data acquisition and application of stimuli were all controlled by Pulse software (Elektronik, Lambrecht, Germany). The filter of the instrument is set to 10kHz (Bessel), and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com