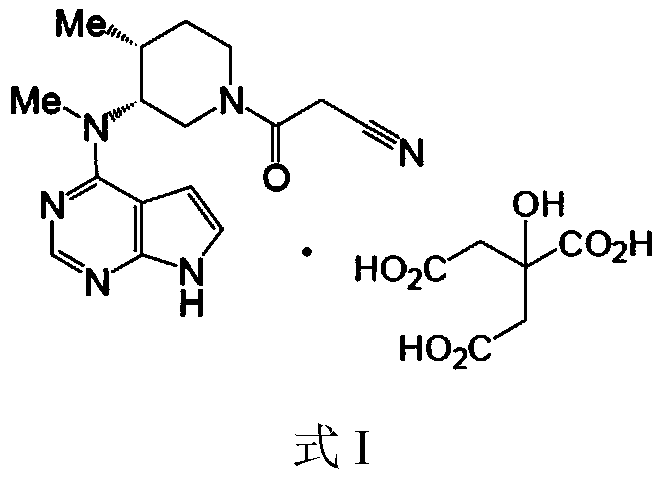
Method for preparingtofacitinib citrate
A technology of tofacitinib and citric acid, which is applied in the field of pharmaceutical preparations, can solve problems such as undisclosed process parameter information, and achieve the effects of reducing the pressure of process quality control, complete reaction, and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
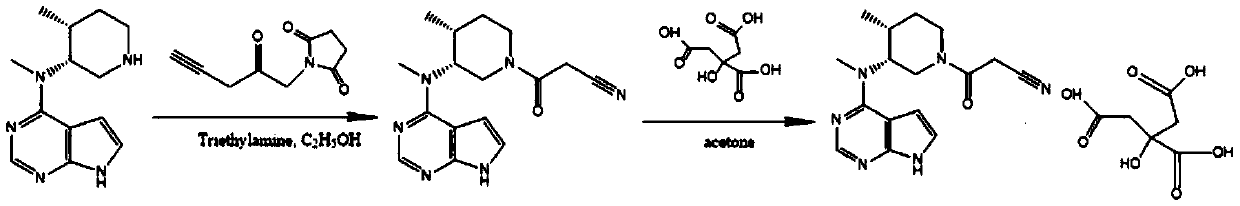
[0037] Step 1: 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)- Preparation of 3-oxopropionitrile
[0038]87g N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, 161g ethyl cyanoacetate and 0.26L of methanol into the reaction vessel, control the temperature at 5-15°C and slowly add 54g of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and then adjust the temperature to 15-15°C Stir the reaction at 20°C for 10 hours, TLC detection (methanol: dichloromethane 1:6) the reaction is almost complete, filter, wash the filter cake with a mixed solvent made of 0.12L water and 0.06L methanol, and dry to obtain a white solid 3-(( 3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropionitrile 100.6 g. Based on N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, the molar yield is 90.8 %. The reaction formula is as follows:
[0039]
[0040] Step 2: 3-((3R,4R)-...
Embodiment 2
[0044] In this embodiment, the solvent ratio in step 2 of embodiment 1 is adjusted.
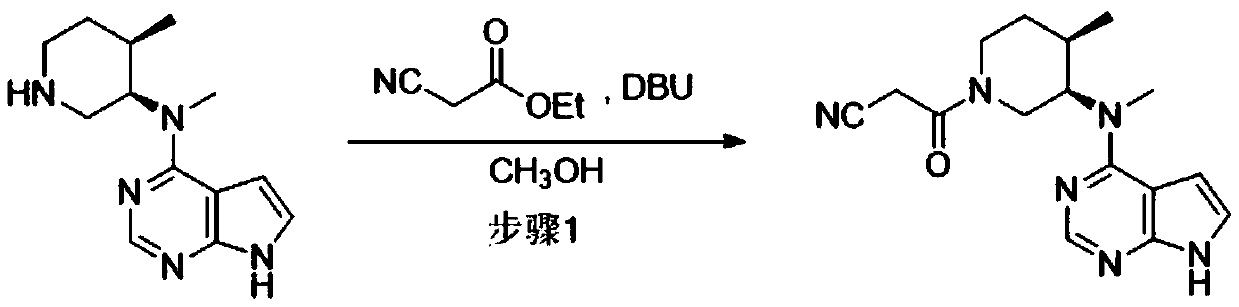
[0045] Step 1: 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)- Preparation of 3-oxopropionitrile
[0046] 87g N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, 161g ethyl cyanoacetate and Put 0.26L of methanol into the reactor, control the temperature at 5-15°C and slowly add 54g of DBU, after the completion, adjust the temperature to 20-25°C for 10 hours, TLC detection (methanol:dichloromethane 1:6) The reaction is basically complete, filter, and use The filter cake was washed with a mixed solvent made of 0.12L purified water and 0.06L methanol, and dried to obtain a white solid 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3 -d] pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropionitrile 101 g. Based on N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, the molar yield is 91.2 %.
[0047] Step 2: 3...
Embodiment 3
[0050] In this embodiment, the solvent ratio in step 2 of embodiment 1 is adjusted.
[0051] Step 1: 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)- Preparation of 3-oxopropionitrile
[0052] 87g N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, 161g ethyl cyanoacetate and Put 0.26L of methanol into the reactor, control the temperature at 5-15°C and slowly add 54g of DBU, after the completion, adjust the temperature to 15-20°C for 8 hours, TLC detection (methanol: dichloromethane 1:6) The reaction is basically complete, filter, and use The filter cake was washed with a mixed solution of 0.12L water and 0.06L methanol, and dried to obtain a white solid 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3- d] pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropionitrile 100.8 g. Based on N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, the molar yield is 91.0 %.
[0053] Step 2: 3-((3R,4R)-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com