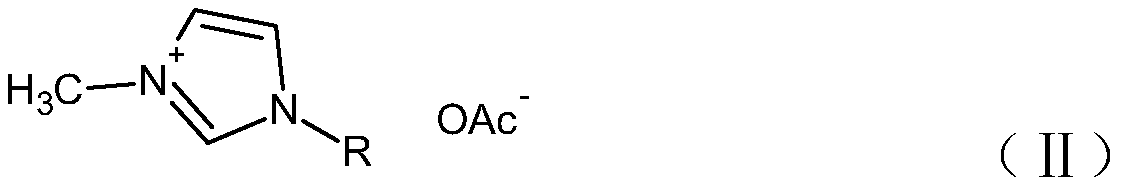Preparing method of high-purity imidazole acetate ionic liquid
A technology of pure imidazole acetate and methylimidazole acetate, which is applied in the field of preparation of high-purity ionic liquids, can solve the problems of harsh reaction conditions, high raw material alkoxide prices, and expensive raw materials, and achieve moderate reaction speed, The effect of high product purity and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of 1-ethyl-3-methylimidazolium acetate ionic liquid:
[0044] Dissolve 1.0mol of 1-ethyl-3-methylimidazolium chloride salt with 48g of water to obtain solution A, dissolve 1.2mol of lithium perchlorate with 254g of water to obtain solution B; mix solution A with solution B, Add 575g of dichloromethane, mechanically stir and react for 12 hours; separate the liquid, separate the water phase as waste liquid, recycle, wash the organic phase with 260g of pure water each time, wash 5 times, use AgNO 3 No precipitation was detected in the solution; the organic phase was rotary evaporated in vacuum at 80°C to obtain 193g of 1-ethyl-3-methylimidazole perchlorate ionic liquid intermediate, with a yield of 91.9%; 579g of ethanol was added, and under vigorous stirring, Slowly add a total of 90.1g of potassium acetate in 4 batches. During the addition process, solids dissolve and precipitate continuously, and continue to stir for 24 hours; stop stirring, freeze to -15°C ...
Embodiment 2
[0046] Preparation of 1-butyl-3-methylimidazolium acetate ionic liquid:
[0047] Dissolve 1.0mol of 1-butyl-3-methylimidazolium bromide with 58g of water to obtain solution A, dissolve 0.8mol of lithium perchlorate with 170g of water to obtain solution B; mix solution A with solution B, Add 531g of dichloromethane, mechanically stir and react for 12 hours; separate the liquid, wash the organic phase with 278g of pure water each time, wash 3 times, use AgNO 3 No precipitation was detected in the solution; the organic phase was vacuum rotary evaporated at 80°C to obtain 178.4g of the 1-butyl-3-methylimidazole perchlorate ionic liquid intermediate, with a yield of 93.7%; 446g of ethanol was added, and under vigorous stirring, Slowly add a total of 79.8g of potassium acetate in 4 batches. During the addition process, solids are continuously dissolved and precipitated. Continue to stir for 12 hours; stop stirring, freeze to -15°C for 36 hours, filter, and spin the filtrate at 80°C....
Embodiment 3
[0049] Preparation of 1-octyl-3-methylimidazolium acetate ionic liquid:
[0050] Dissolve 1.0 mol of 1-octyl-3-methylimidazolium chloride salt with 78 g of water to obtain solution A, dissolve 2.0 mol of lithium perchlorate with 426 g of water to obtain solution B; mix solution A with solution B, Add 841g of dichloromethane, mechanically stir and react for 12 hours; separate the liquid, separate the water phase as waste liquid, recycle, wash the organic phase with 256g of pure water each time, wash 3 times, use AgNO 3 The solution detects no precipitation; the organic phase is vacuum rotary evaporated at 80°C to obtain 193g of the 1-octyl-3-methylimidazole perchlorate ionic liquid intermediate, with a yield of 91.9%; add 965g of ethanol, stir vigorously, and divide Slowly add a total of 90.1g of potassium acetate in 4 batches. During the addition process, solids dissolve and precipitate continuously, and continue to stir for 48 hours; stop stirring, freeze to -15°C for 12 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com