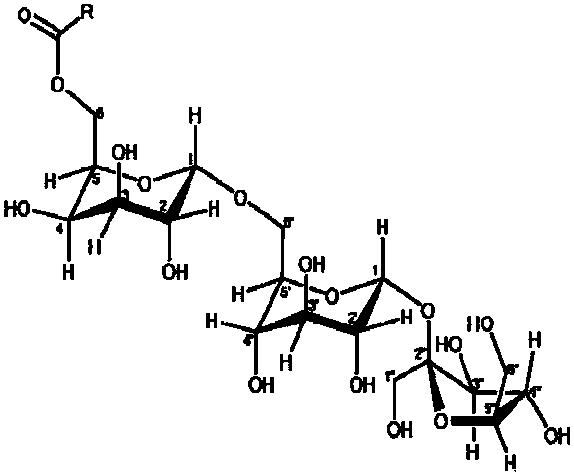
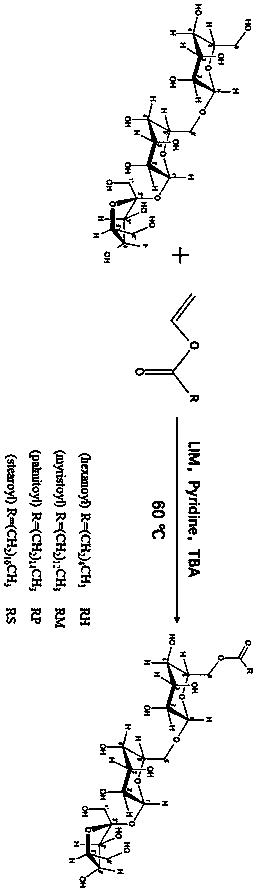
6-O-acyl raffinose monoester and synthesis method thereof
A technology of acyl raffinose monoester and acyl raffinose, which is applied in the field of 6-O-acyl raffinose monoester and its synthesis, and can solve the practical application of raffinose monoester synthesis route. Research and other issues to achieve the effect of shortening time-consuming, optimizing process conditions and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 6-O-hexanoyl raffinose monoester (RH) synthesis method such as figure 2 That is, the synthesis method of 6-O-acyl raffinose monoester of the present invention is shown in the flow chart. The synthesis method selects vinyl caproate as the reactant, and is separated by enzymatic reaction and fast chromatography to change the molecular weight and structure of the sugar ester. , Specifically including the following steps and process conditions:
[0036] Step 1: Enzymatic reaction
[0037] (1) After adding 45 mL of pyridine, 55 mL of tert-butanol (9:11, v / v) and magnets in turn, treat the mixture obtained with 1 g of lipase TLIM and 4 mmol of D-raffinose, and magnetically stir at 50°C for 5 minutes , Treat the mixture with vinyl caproate, the molar mass ratio of the raffinose to vinyl caproate is 1:5, that is, add 20 mmol of vinyl caproate;
[0038] (2) The resulting mixture was magnetically stirred at 50°C for 14 hours, until all D-raffinose had been consumed by TLC analysis, co...
Embodiment 2
[0047] The synthesis method of 6-O-myristoyl raffinose ester (RM) is as figure 2 That is to say, as shown in the flow chart of the 6-O-acyl raffinose monoester synthesis method of the present invention, the synthesis method selects vinyl myristate as the reactant, and undergoes enzymatic reaction and fast chromatographic elution separation to change the molecular weight of the sugar ester And structure, specifically including the following steps and process conditions:
[0048] Step 1: Enzymatic reaction
[0049] (1) After adding 45 mL of pyridine, 55 mL of tert-butanol (9:11, v / v) and magnets in sequence, treat the mixture obtained with 1 g of lipase TLIM and 4 mmol of D-raffinose, and magnetically stir at 55°C for 8 minutes The molar mass ratio of the raffinose to vinyl myristate is 1:6, that is, 24 mmol of vinyl myristate is added, and the resulting mixture is magnetically stirred at 55°C for 14 hours, until all D is determined by TLC analysis. -Raffinose has been consumed, co...
Embodiment 3
[0057] 6-O-palmitoyl raffinose monoester (RP) synthesis method such as figure 2 That is, as shown in the flow chart of the 6-O-acyl raffinose monoester synthesis method of the present invention, the synthesis method selects vinyl palmitate as the reactant, and undergoes enzymatic reaction and fast chromatographic elution separation to change the molecular weight and structure of the sugar ester. Specifically include the following steps and process conditions:
[0058] Step 1: Enzymatic reaction
[0059] After adding 45 mL of pyridine, 55 mL of tert-butanol (9:11, v / v) and magnets in sequence, the mixture obtained by treating with 1 g of lipase TLIM and 4 mmol of D-raffinose was magnetically stirred at 55° C. for 5 minutes. The molar mass ratio of raffinose to vinyl palmitate is 1:5, that is, 20 mmol of vinyl palmitate is added, and the resulting mixture is magnetically stirred at 55°C for 14 hours, until all D-raffinose has been determined by TLC analysis. After being consumed, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com