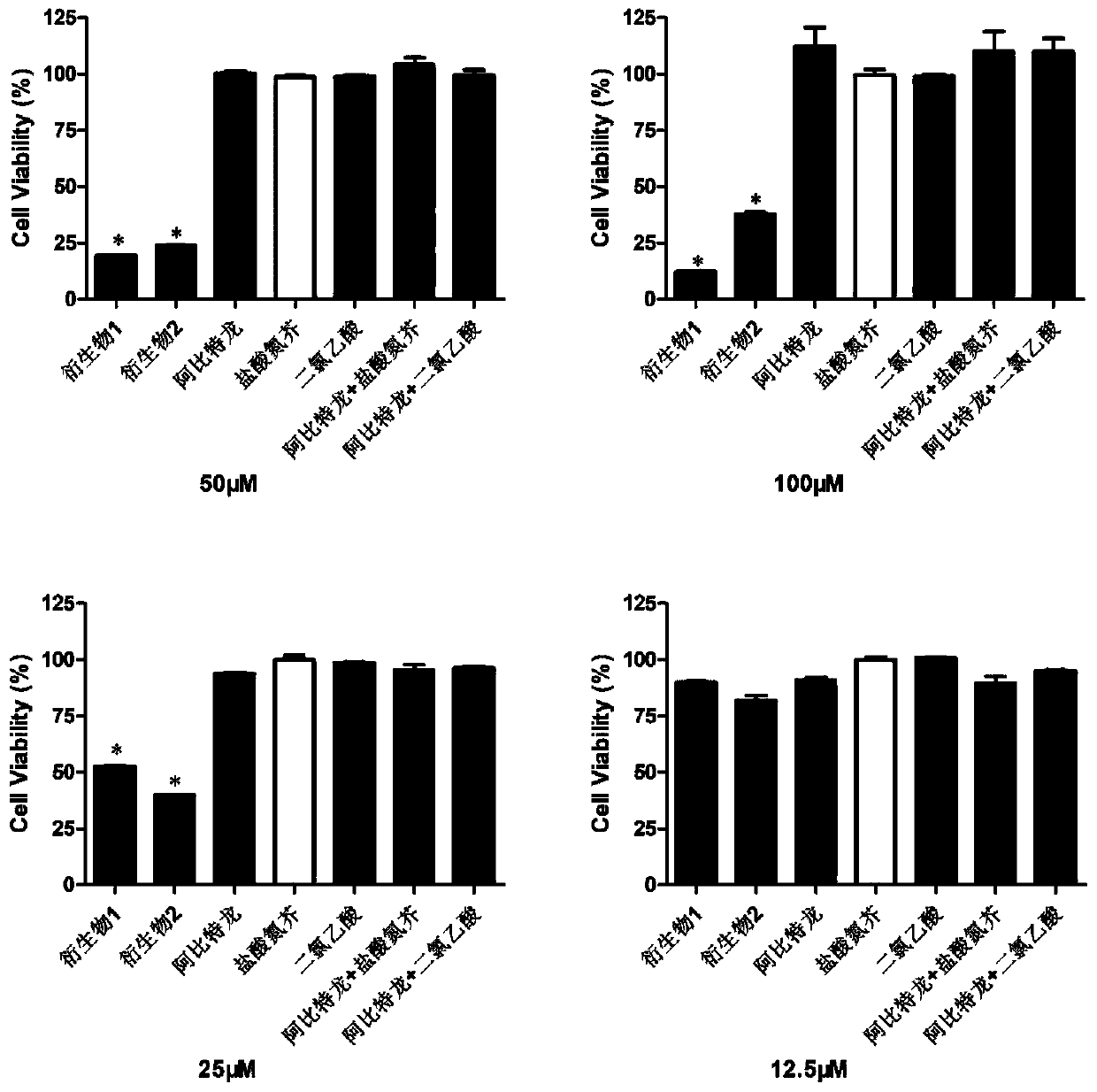
Abiraterone derivative and preparation method and application thereof
A technology of abiraterone and derivatives, applied in the field of preparation of abiraterone derivatives, can solve the problems of low activity of prostate cancer cells and limited clinical treatment effect of abiraterone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
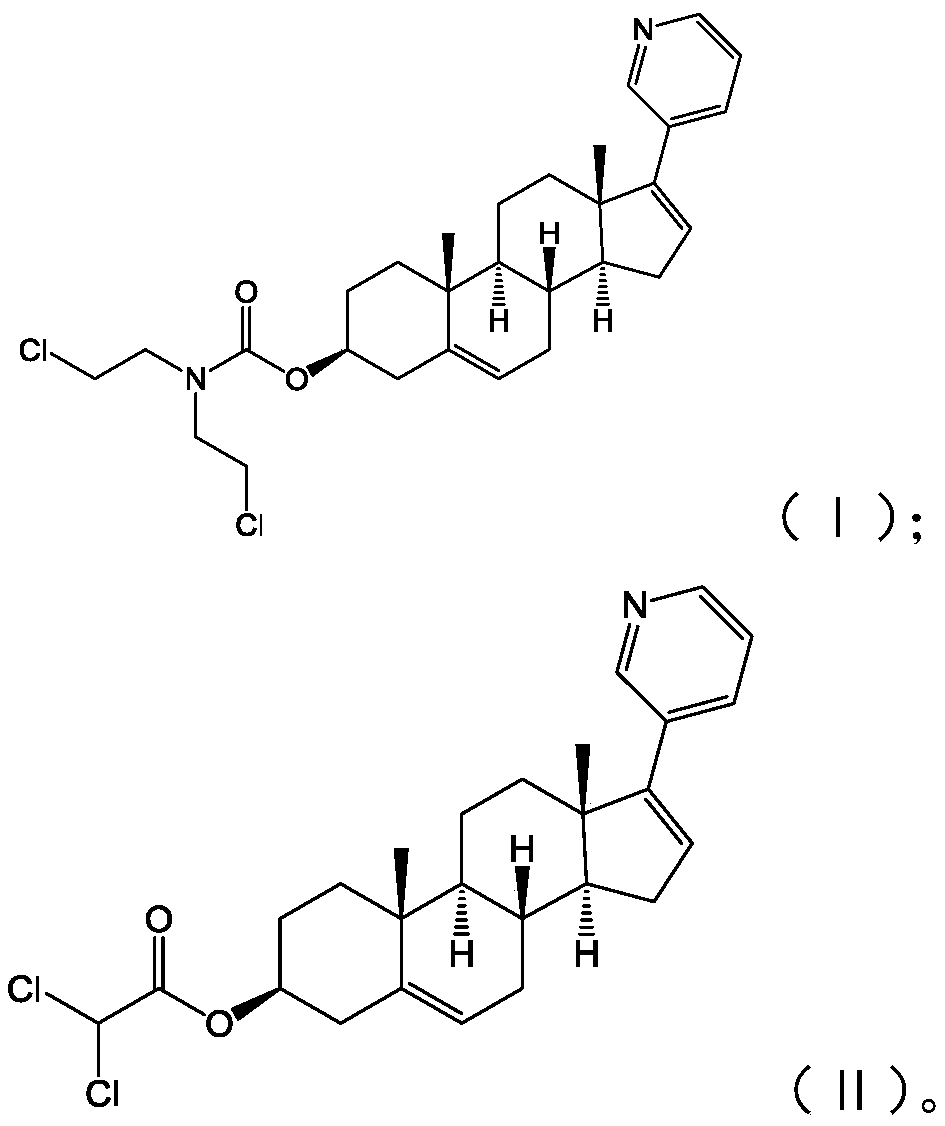
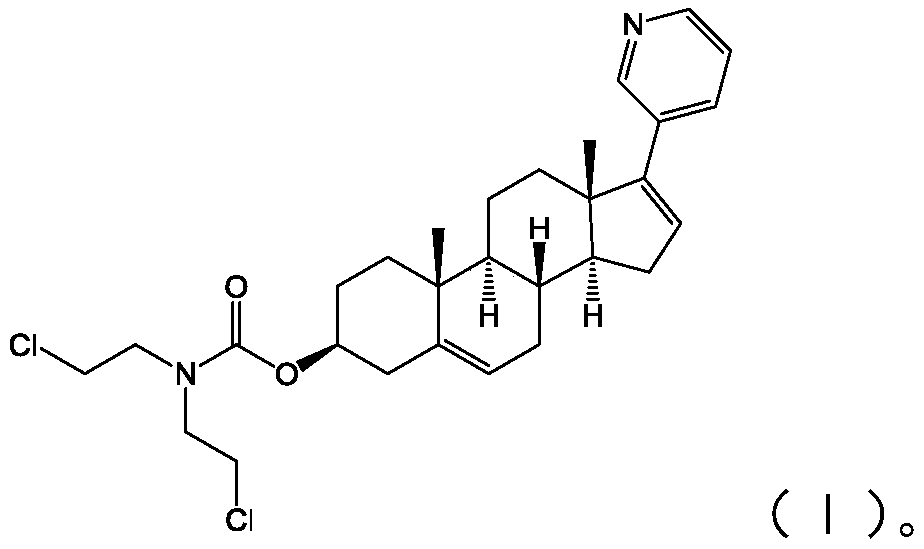
[0028] The present application also provides a preparation method of the abiraterone derivative shown in formula (I), including:
[0029] Abiraterone and solid phosgene are reacted in a solvent to obtain an intermediate product;
[0030] The intermediate product is reacted with dichloroethylamine hydrochloride to obtain the abiraterone derivative shown in formula (I);
[0031]
[0032] In the above process of preparing the abiraterone derivatives shown in formula (I), first, abiraterone and solid phosgene are initially reacted under alkaline conditions in a solvent to obtain an intermediate, and the solvent is dichloromethane , the base is triethylamine; after the above reaction is complete, dichloroethylamine hydrochloride is added, and the abiraterone derivative shown in formula (I) is obtained after the reaction. In above-mentioned reaction process, the mol ratio of described Abiraterone and described solid phosgene is 1:(1~2.5); The mol ratio of described intermediate ...
Embodiment 1
[0045] Embodiment 1 Preparation of Abiraterone Derivative 1
[0046] Add 1 gram of abiraterone (commercially available, HPLC purity 98%) to 40 milliliters of DCM, 0.8 grams of triethylamine, cool to 0-5 degrees, slowly add 1.5 grams of solid phosgene, stir for 1-2 hours, and confirm the raw material by TLC The reaction is complete; then add 0.5 g of dichloroethylamine hydrochloride, stir for 4-5 hours, TLC monitors to confirm the complete reaction of the raw materials, then add 50 ml of water and DCM, stir for 2 min, separate the liquids, add 50 ml of sodium bicarbonate aqueous solution to wash Organic phase, concentrated organic phase, 10-20 ml of ethyl acetate, stirred and crystallized to obtain 0.7 g of white solid.
[0047] 1 HNMR (400MHz, CDCl3): δ8.62 (d, J = 2.1Hz, 1H), 8.45 (dd, J = 4.8, 1.5Hz, 1H), 7.64 (dt, J = 7.9, 2.0Hz, 1H), 7.21 (dd,J=7.9,4.8Hz,1H), 5.99(dd,J=3.4,1.8Hz,1H), 5.41-5.37(m,1H), 4.5(m,1H), 3.6-3.7(m,8H ), 2.36-2.20(m,3H), 2.12-2.00(m,3H), 1.89-1.81...
Embodiment 2
[0050] Embodiment 2 Preparation of Abiraterone Derivative 2
[0051] Add 1 gram of Abiraterone to 40 milliliters of DCM, add 0.8 grams of triethylamine, cool down to 0-5 degrees, slowly add 0.5 grams of dichloroacetyl chloride dropwise, stir for 5-6 hours after dripping, add 50 milliliters of water, and stir After 2 minutes, the organic phase was washed once with 50 ml of sodium bicarbonate solution, dried, and the organic phase was concentrated, and the residue was passed through a silica gel chromatography column with ethyl acetate:petroleum ether=1:1 eluent to obtain 0.5 g of the product.
[0052] 1HNMR (400MHz, CDCl3): δ8.62 (d, J = 2.1Hz, 1H), 8.45 (dd, J = 4.8, 1.5Hz, 1H), 7.64 (dt, J = 7.9, 2.0Hz, 1H), 7.21 (dd, J=7.9, 4.8Hz, 1H), 6.2(m, 1H), 5.99(dd, J=3.4, 1.8Hz, 1H), 5.41-5.37(m, 1H), 4.6(m, 1H), 2.36 -2.20(m,3H), 2.12-2.00(m,3H), 1.89-1.81(m,2H), 1.80-1.43(m,7H), 1.15-1.10(m,2H), 1.07(s,3H) , 1.05(s,3H);
[0053] ESI-MS m / z:460.44[M+H] + .
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com