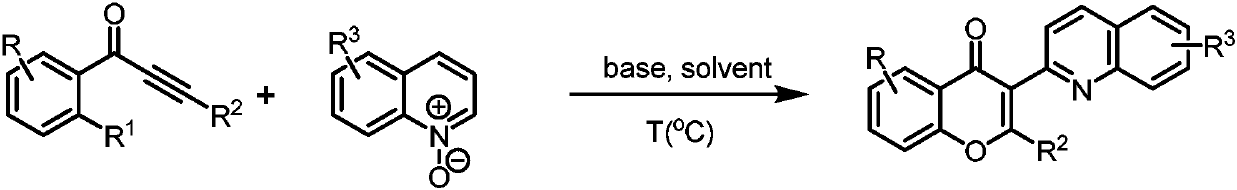Preparation method for 2,3-disubstituted benzo-gamma-pyrone derivative
A technology of pyrone and disubstitution, applied in 2 fields, can solve the problems of low efficiency, not enough environmental protection, cumbersome steps, etc., and achieve the effect of moderate reaction time, mild reaction conditions and wide substrate range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
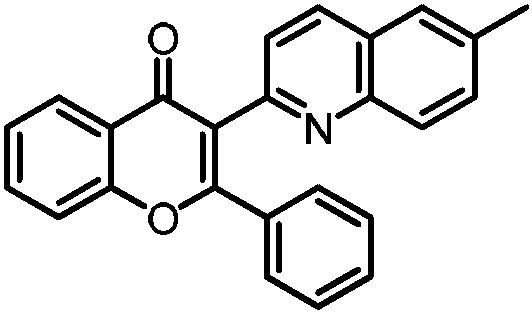
Embodiment 1
[0026] 3-(6-methylquinolin-2-yl)-2-phenyl-4H-chromen-4-one
[0027]
[0028] Add 0.1mmol of the corresponding substituted alkyne ketone, 0.2mmol of 3-methylquinoline 1-oxide, 0.3mmol of sodium phosphate, and 0.5mL of N,N-dimethylformamide into a 15mL reaction tube, and place at 100°C In an oil bath, react under air atmosphere for 12h; cool to room temperature, dilute the reaction solution with ethyl acetate, wash with water three times, organic phase Na 2 SO 4 Dry, filter, concentrate, and purify by column chromatography to obtain 32.8 mg of the target product with a yield of 90%. The NMR and high-resolution mass spectrometry of this target product are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ8.22(m, J=8.0, 1.7Hz, 1H), 7.97(d, J=8.4Hz, 1H), 7.76(d, J=8.6Hz, 1H), 7.63(m, J=8.7, 7.2 , 1.7Hz, 1H), 7.48(m, 2H), 7.39(m, J=8.6, 1.9Hz, 1H), 7.36(d, J=8.3Hz, 2H), 7.33(m, 2H), 7.19(m , 1H), 7.11(m, 2H), 2.44(s, 3H); 13 C NMR (126MHz, CDCl 3 )δ 177.3, 163.2, 156.1, ...
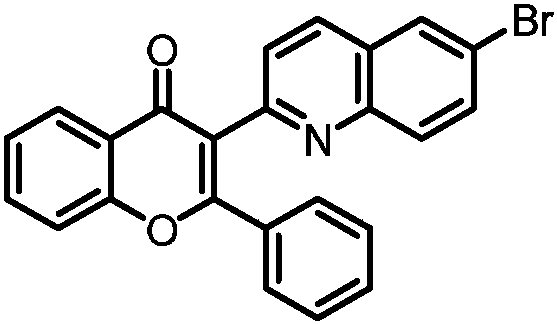
Embodiment 2
[0030] 3-(6-Bromoquinolin-2-yne)-2-phenyl-4H-chromen-4-one
[0031]
[0032] Add 0.1mmol of the corresponding substituted alkyne ketone, 0.2mmol of 3-bromoquinoline 1-oxide, 0.3mmol of sodium phosphate, and 0.5mL of N,N-dimethylformamide into a 15mL reaction tube, and place in a 100°C In an oil bath, react under an air atmosphere for 12 h; cool to room temperature, dilute the reaction solution with ethyl acetate, wash three times with water, and remove the organic phase with Na 2 SO 4 Dry, filter, concentrate, and purify by column chromatography to obtain 26.7 mg of the target product with a yield of 63%. The NMR and high-resolution mass spectrometry of this target product are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ8.23(m, 1H), 7.98(d, J=8.4Hz, 1H), 7.89(d, J=2.1Hz, 1H), 7.72(d, J=9.0Hz, 1H), 7.64(m, 2H), 7.50(d, J=8.4Hz, 1H), 7.44(d, J=8.5Hz, 1H), 7.38(m, 1H), 7.31(m, 2H), 7.22(m, 1H), 7.13( m,2H); 13 C NMR (126MHz, CDCl 3 )δ177.2, 163.5, 156.1, 154.1, ...
Embodiment 3
[0034] 3-(5-Bromoisoquinolin-1-yl)-2-phenyl-4H-chromen-4-one
[0035]
[0036] Add 0.1mmol of the corresponding substituted alkyne ketone, 0.2mmol of 5-bromoisoquinoline 2-oxide, 0.3mmol of sodium phosphate, and 0.5mL of N,N-dimethylformamide into a 15mL reaction tube, and place at 100°C In an oil bath, react under air atmosphere for 12h; cool to room temperature, dilute the reaction solution with ethyl acetate, wash with water three times, organic phase Na 2 SO 4 Dry, filter, concentrate, and purify by column chromatography to obtain 18.1 mg of the target product with a yield of 43%. The NMR and high-resolution mass spectrometry of this target product are characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ8.54(d, J=5.9Hz, 1H), 8.21(m, 1H), 7.93(d, J=5.9Hz, 1H), 7.86(d, J=7.4Hz, 1H), 7.80(d, J=8.3Hz, 1H), 7.69(m, 1H), 7.55(d, J=8.4Hz, 1H), 7.40(t, J=7.5Hz, 1H), 7.25(m, 3H), 7.19(d, J=6.4Hz, 1H), 7.09(t, J=7.7Hz, 2H); 13 C NMR (126MHz, CDCl 3 )δ 177.2, 163.5, 156.3, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com