A thermally active delayed fluorescence organic compound with 2-cyanopyrazine as acceptor and its preparation and application
A technology of organic compounds and delayed fluorescence, which is applied in the fields of organic chemistry, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., and can solve problems such as spectrum being affected by solvent polarity, large excited state dipole moment, and device stability degradation , to achieve good industrial application prospects, good photoelectric performance, and reduce the effect of impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of compound 19TCzPZCN:
[0043] synthetic route:
[0044]
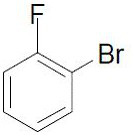
[0045] (1) Add 2.79g (10.0mmol) 3,6-di-tert-butylcarbazole and 6.52g (20.0mmol) cesium carbonate into a 100mL three-necked flask, fill with nitrogen for 3 times to remove oxygen, and inject 1.31mL under nitrogen atmosphere (12mmol) o-fluorobromobenzene, 15mL DMF ultra-dry solvent, heated to reflux at 160 degrees Celsius for 24h. After the reaction was monitored by TCL, the reaction system was cooled to room temperature, extracted with 150 mL of ethyl acetate and 300 mL of saturated brine, then washed with saturated brine (100 mL×3) for 3 times, dried over anhydrous sodium sulfate for 10 min, filtered, concentrate. The crude product was passed through a silica gel column (5×10 cm), and the pure product was isolated and dried in vacuo to obtain 4.01 g of product 19-1 with a yield of 92%.
[0046] 1 H NMR (400MHz, CDCl3): δ=8.14(s, 2H), 7.84(d, J=8Hz, 1H), 7.51-7.31(m, 5H), 6.99(...
Embodiment 2
[0051] Embodiment 2: the synthesis of compound 24 2TCzPZCN:
[0052]
[0053] (1) Add 2.79g (10.0mmol) 3,6-di-tert-butylcarbazole, 6.52g (20.0mmol) cesium carbonate into a 100mL three-necked flask, fill with nitrogen for 3 times to remove oxygen, and inject 0.78g under nitrogen atmosphere (4mmol) 2,5-difluorobromobenzene, 15mL DMF ultra-dry solvent, heated to reflux at 160°C for 24h. After the reaction was monitored by TCL, the reaction system was cooled to room temperature, extracted with 150 mL of ethyl acetate and 300 mL of saturated brine, then washed with saturated brine (100 mL×3) for 3 times, dried over anhydrous sodium sulfate for 10 min, filtered, concentrate. The crude product was passed through a silica gel column (5×10 cm), the pure product was isolated, and vacuum-dried to obtain 2.59 g of product 24-1 with a yield of 91%.
[0054] 1 H NMR (400MHz, CDCl 3 ): δ=8.18-8.17(m, 4H), 8.11(d, J=4Hz, 1H), 7.75-7.72(m, 1H), 7.63(d, J=12Hz, 1H), 7.54(m, 4H) ,7.52(d,...
Embodiment 3
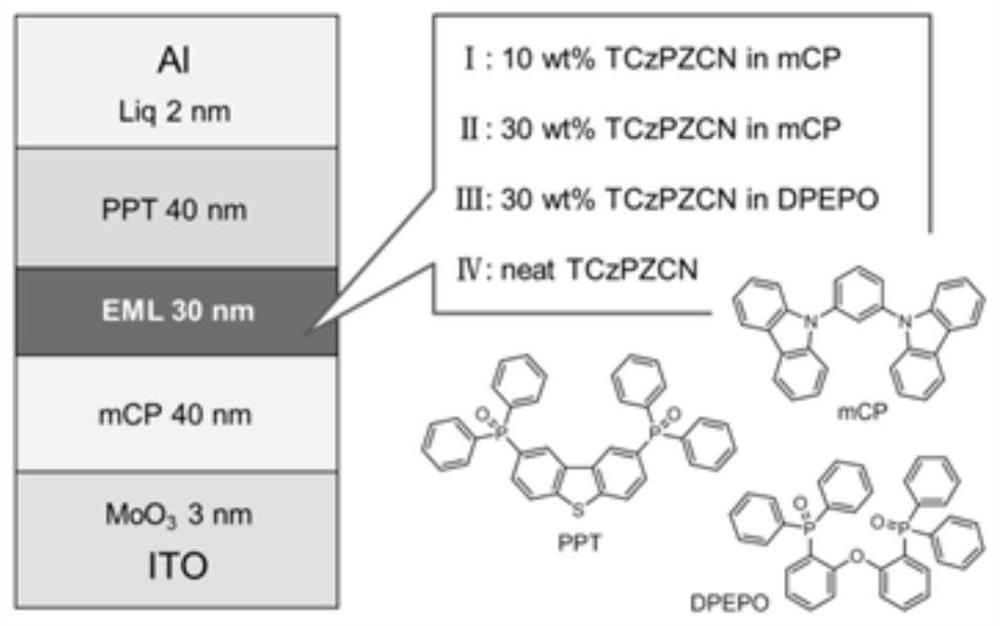
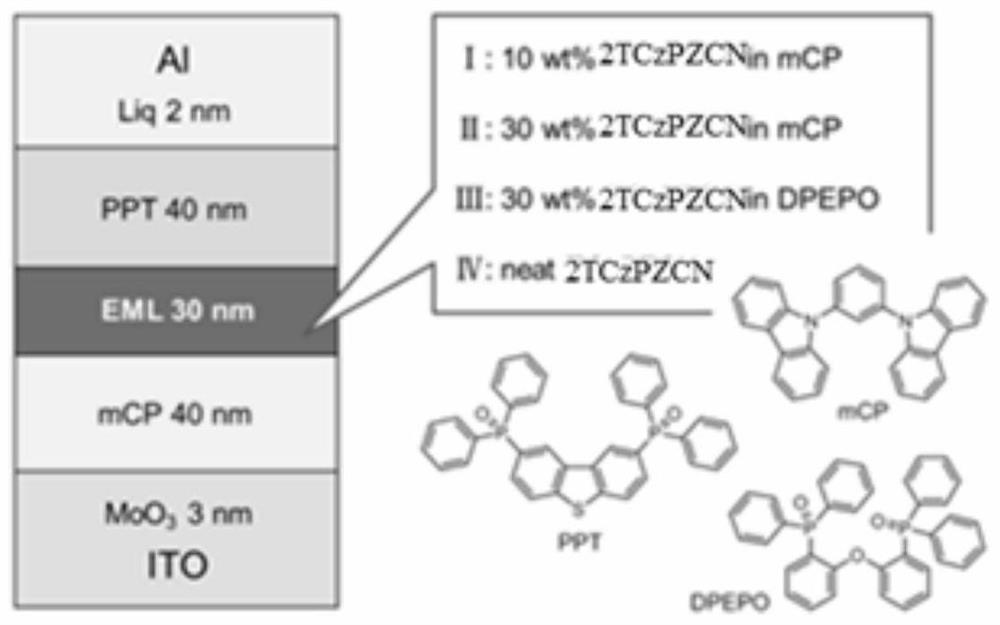
[0063] light emitting devices such as figure 1 As shown, it specifically includes: transparent substrate layer 1 / ITO anode layer 2 / hole injection layer 3 (MoO 3 , thickness 3nm) / hole transport layer 4 (mCP, thickness 40nm) / light-emitting layer 5 (mCP or DPEPO and compound 19 are mixed according to the weight ratio of 10~30:90~70, thickness 30nm) / electron transport layer 6 ( PPT, thickness 40nm) / electron injection layer 7 (Liq, thickness 2nm) / cathode reflective electrode layer 8 (Al, thickness 10nm). The structural formulas of the materials involved are as follows:
[0064]
[0065] Concrete preparation process is as follows:
[0066] The transparent substrate layer 1 is a transparent substrate, such as transparent PI film, glass and the like. The ITO anode layer 2 (with a film thickness of 150nm) was washed, that is, alkali washing, pure water washing, drying, and ultraviolet-ozone washing were performed in order to remove organic residues on the transparent ITO surface....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com