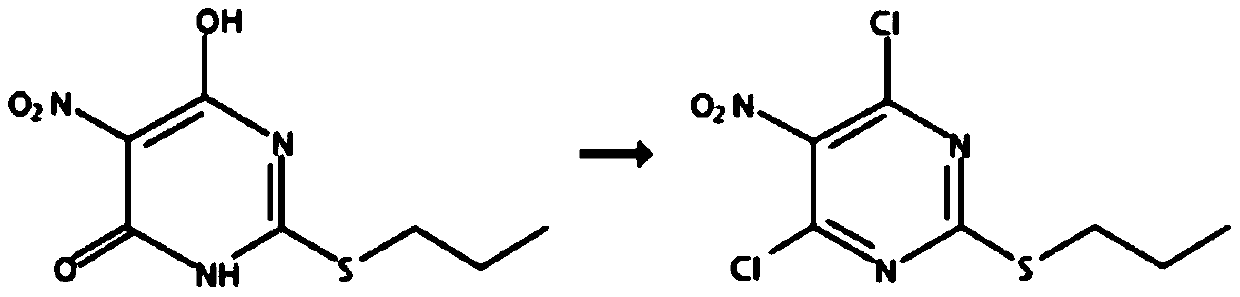
Method for preparing 4,6-dichloro-5-nitro-2-(propylmercapto)-pyrimidine
A technology of propylmercapto and nitro, which is applied in the field of preparation of 4,6-dichloro-5-nitro-2--pyrimidine, can solve the problems of large environmental pollution, harsh reaction conditions, and low reaction yield, and achieve The effect of low operating cost and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
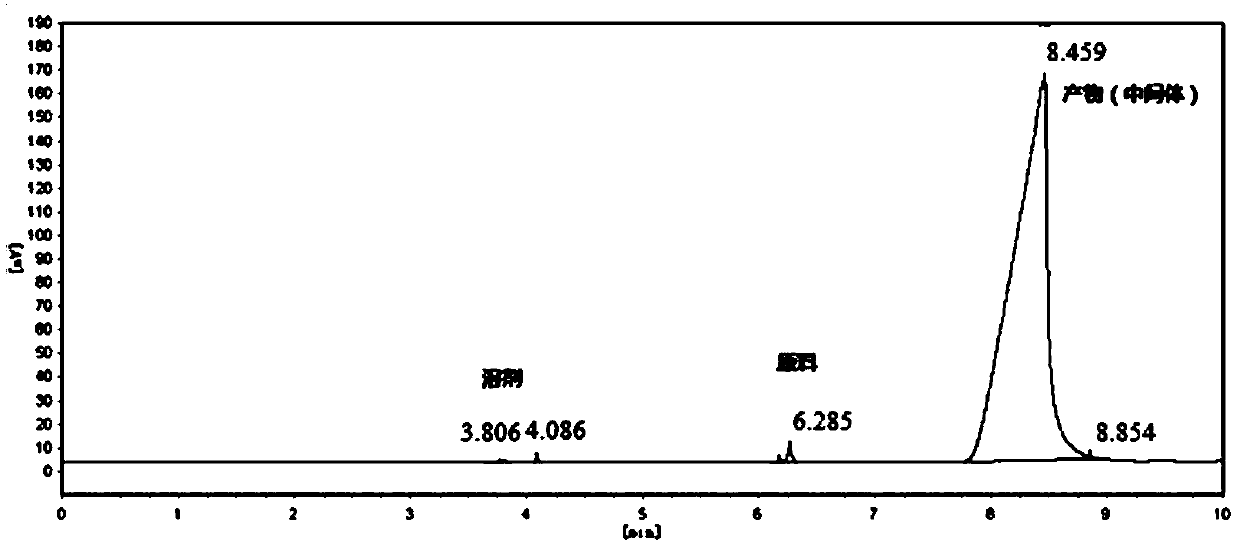
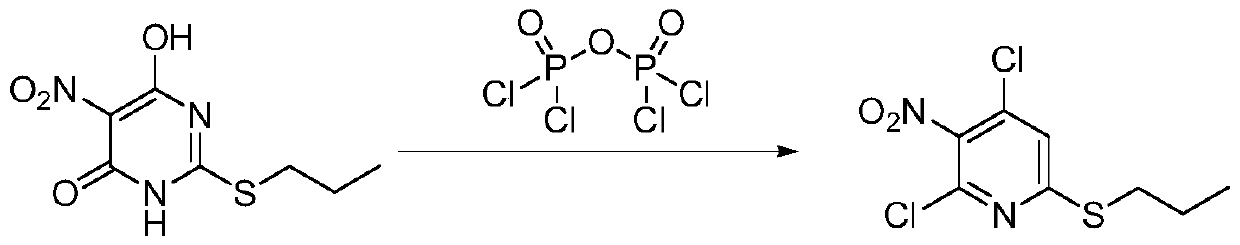
[0021] Add 250 milliliters of toluene in a 500 milliliter three-necked flask, add 50.0 grams of 6-hydroxyl-5-nitro-2-(propylmercapto)-4(3H)-pyrimidinone (purity: 98+%, CAS number: 145783 -12-6, alias: 4,6-dihydroxy-5-nitro-2-(propylmercapto)pyrimidine,), dissolved in toluene, cooled to 0°C, and 27.0 grams of pyrophosphorus was added dropwise within 2 hours Acyl chloride, heat up to 80°C, add 0.13 g of monoethanolamine, react for 10 hours, after the reaction, cool down to 0°C, pour into ice water, stir for 1 hour, separate layers, add 60-120 mesh neutral The silica gel was stirred for 0.5 hours, filtered, and the filtrate was rotatively evaporated at 50°C to recover toluene to obtain 45.8 grams of oily 4,6-dichloro-5-nitro-2-(propylmercapto)-pyrimidine, the content of which was analyzed by gas chromatography, and the content was 97.5% ( attached figure 1 ). Gas chromatograph: SHIMADZU GC-2014C. Analysis conditions: HP-1 capillary column, 15m*0.53mm, 0.15μm, initial temperatu...
Embodiment 2
[0024] Add 250 milliliters of toluene to a 500 milliliter three-necked flask, add 50.0 grams of 6-hydroxy-5-nitro-2-(propylmercapto)-4(3H)-pyrimidinone, dissolve in toluene, cool to 20°C, Add 54 grams of pyrophosphoryl chloride dropwise within 8 hours, heat up to 110°C, add 2.27 grams of diethanolamine, and react for 40 hours. After the reaction, cool down to 30°C, pour into ice water, stir for 2 hours, and separate layers. Add 60-120 mesh neutral silica gel to the upper toluene layer and stir for 1 hour, filter, the filtrate is 70°C rotary evaporation to recover toluene, and the oily product 4,6-dichloro-5-nitro-2-(propylmercapto)-pyrimidine 49.2 grams, content 98.1%.
Embodiment 3
[0026] Add 250 milliliters of toluene to a 500 milliliter three-necked flask, add 50.0 grams of 6-hydroxy-5-nitro-2-(propylmercapto)-4(3H)-pyrimidinone, dissolve in toluene, cool to 0°C, Add 40.0 grams of pyrophosphoryl chloride dropwise within 2 hours, heat up to 80°C, add 3.20 grams of triethanolamine, and react for 10 hours. After the reaction, cool down to 0°C, pour into ice water, stir for 1 hour, and separate layers. Add 60-120 mesh neutral silica gel to the upper toluene layer and stir for 0.5 hours, filter, and the filtrate is rotary evaporated at 50°C to recover toluene, and the oily product 4,6-dichloro-5-nitro-2-(propylmercapto)-pyrimidine 50.5 grams, content 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com