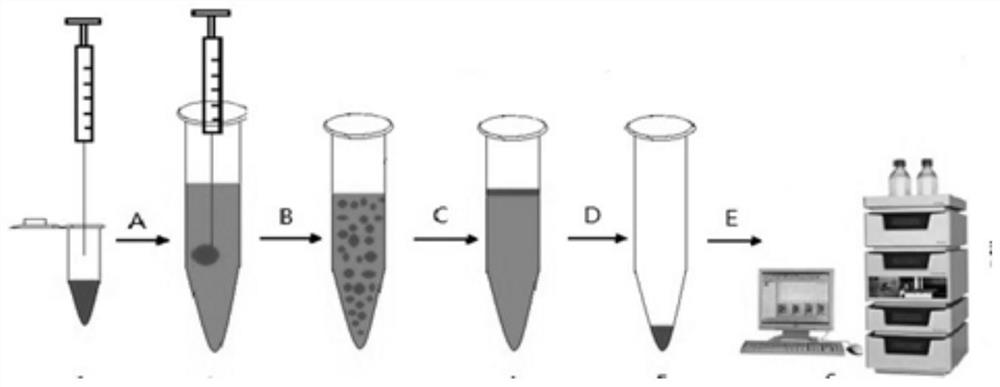
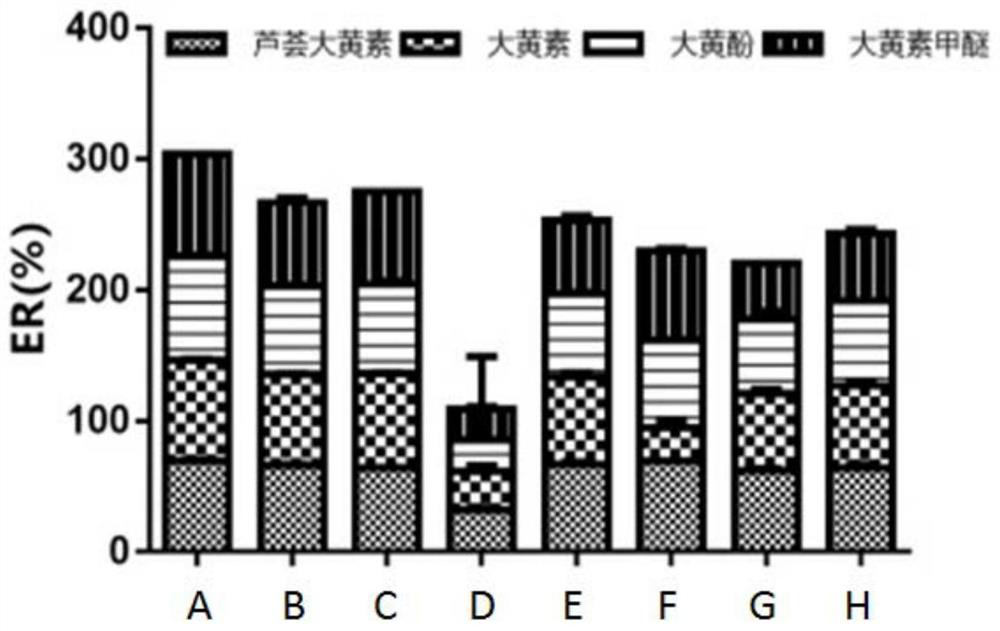
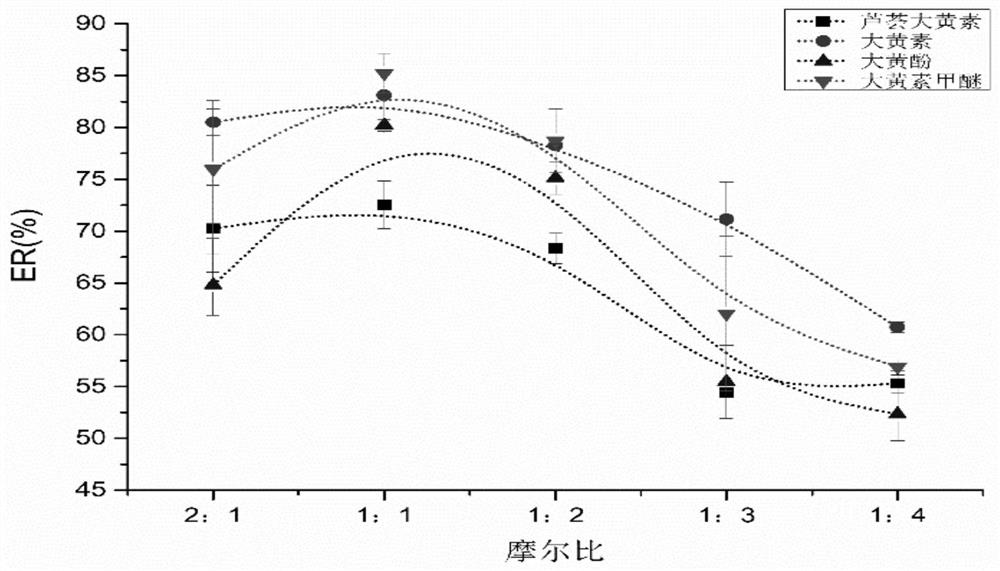
A kind of detection reagent and detection method of anthraquinone compounds
A detection method and detection reagent technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of high cost of mass spectrometry detection of anthraquinone substances, and achieve the effects of short test period, less centrifugation and filtration, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This example provides a detection reagent A for anthraquinone compounds, the detection reagent includes a hydrogen bond acceptor and a hydrogen bond donor corresponding to the hydrogen bond acceptor, wherein the hydrogen bond acceptor is L-menthol, and the hydrogen bond donor The monomer is acetic acid, and the molar ratio of hydrogen bond acceptor and hydrogen bond donor is 1:(0.5~4).
Embodiment 2
[0041] This example provides a detection reagent B for anthraquinone compounds. The detection reagent includes a hydrogen bond acceptor and a hydrogen bond donor corresponding to the hydrogen bond acceptor, wherein the hydrogen bond acceptor is L-menthol, and the hydrogen bond donor is L-menthol. The body is lactic acid, and the molar ratio of hydrogen bond acceptor and hydrogen bond donor is 1:(0.5~4).
Embodiment 3
[0043]This example provides a detection reagent C for anthraquinone compounds. The detection reagent includes a hydrogen bond acceptor and a hydrogen bond donor corresponding to the hydrogen bond acceptor, wherein the hydrogen bond acceptor is L-menthol, and the hydrogen bond donor is L-menthol. The monomer is 1,3-butanediol, and the molar ratio of hydrogen bond acceptor and hydrogen bond donor is 1:(0.5-4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com