Styrene-bisphenanthroimidazole derivative, preparation method therefor and application of styrene-bisphenanthroimidazole derivative
A technology for phenanthroimidazole and derivatives, which is applied in the field of styrene-bisphenanthroimidazole derivatives and its preparation, can solve the problems of low fluorescence quantum yield, poor photothermal stability, and low luminous intensity, and achieve high fluorescence quantum The effect of good yield, good stability, and strong fluorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Preparation of Intermediate A
[0057] Mix and dissolve 3.9g of 4-bromo-benzophenone, 4.41g of zinc powder and 50mL of tetrahydrofuran, stir magnetically under the protection of nitrogen, cool to 0°C, inject 4.4ml of titanium tetrachloride, and Stir at -10°C for 30 min, then magnetically stir at 90°C under the protection of nitrogen for a reaction time of 20 h, cool to room temperature, and add 30 ml of dilute hydrochloric acid to quench the reaction. After the reaction, it was purified by extraction and column chromatography (eluent: petroleum ether / dichloromethane) to obtain 3.32 g of Intermediate A.
[0058] The reaction equation is as follows:
[0059]
[0060] 2. Preparation of Intermediate B
[0061] Weigh 1.7g of (4-bromobenzyl) diethyl phosphonate, 1.3g of 4-bromo-diphenmethanone and 130mg of potassium tert-butoxide, add 40mL of tetrahydrofuran, and dissolve to obtain a mixture. Magnetic stirring was carried out at a temperature of °C under the protecti...
Embodiment 2
[0093] 1. Preparation of Intermediate A
[0094] Mix and dissolve 3.9g of 4-bromo-benzophenone, 4.41g of zinc powder and 50mL of tetrahydrofuran, stir magnetically under the protection of nitrogen, cool to 0°C, inject 4.4ml of titanium tetrachloride, and Stir at -10°C for 30 min, then magnetically stir at 90°C under the protection of nitrogen for a reaction time of 20 h, cool to room temperature, and add 30 ml of dilute hydrochloric acid to quench the reaction. After the reaction, it was purified by extraction and column chromatography (eluent: petroleum ether / dichloromethane) to obtain 3.32 g of Intermediate A.
[0095] 2. Preparation of Intermediate B
[0096] Weigh (4-bromobenzyl) diethyl phosphonate, 4-bromo-diphenmethanone and 130 mg of potassium tert-butoxide, control (4-bromobenzyl) diethyl phosphonate and 4-bromo-dibenzone The molar ratio of benzophenone is 1:1.5; add 40mL of tetrahydrofuran and dissolve to obtain a mixture, which is magnetically stirred at a temperatu...
Embodiment 3
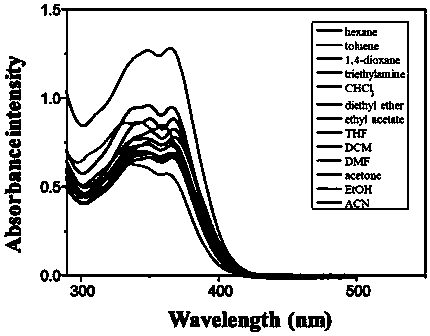
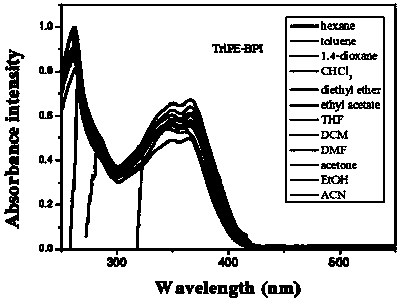
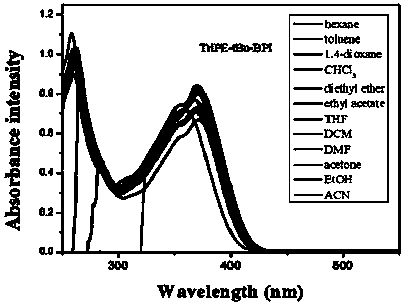
[0112] 1. Structural identification of tetraphenylethylene-bisphenanthroimidazole derivatives (BUBPI) and two triphenylethylene-bisphenanthroimidazole derivatives (TriPE-BPI and TriPE-tBu-BPI)
[0113] The hydrogen signals of the products BUBPI, TriPE-BPI and TriPE-tBu-BPI were scanned by nuclear magnetic resonance and their hydrogen signals were identified. The results are as follows Figure 1 to Figure 3 shown.
[0114] Depend on figure 1 It can be seen that the characteristic wave numbers of tetraphenylethylene-bisphenanthroimidazole derivatives (BUBPI) are 8.82, 8.69, 8.62, 7.66, 7.55, 7.40, 7.29, 7.20, 7.16, 7.05, 1.37.
[0115] Depend on figure 2 It can be seen that R 2 , R 3 The characteristic wave numbers of triphenylethylene-bisphenanthroimidazole derivatives (TriPE-BPI) with both H are 8.85, 8.70, 8.63, 7.68, 7.50, 7.25, 7.06, 6.93.
[0116] Depend on image 3 It can be seen that R 2 , R 3 The characteristic wave numbers of triphenylethylene-bisphenanthroimi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com