Pyridine/bipyridine conjugated micro-porous polymer, preparation method and application thereof
A technology of conjugated micropores and bipyridyl, which is applied in separation methods, chemical instruments and methods, educts, etc., can solve the problems of uncertainty in pyridine nitrogen content, harsh synthesis conditions, and the need for heavy metal catalysis, etc., and achieve the goal of increasing CO2 The effect of adsorption capacity and CO2/N2 selectivity, increasing nitrogen content, and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
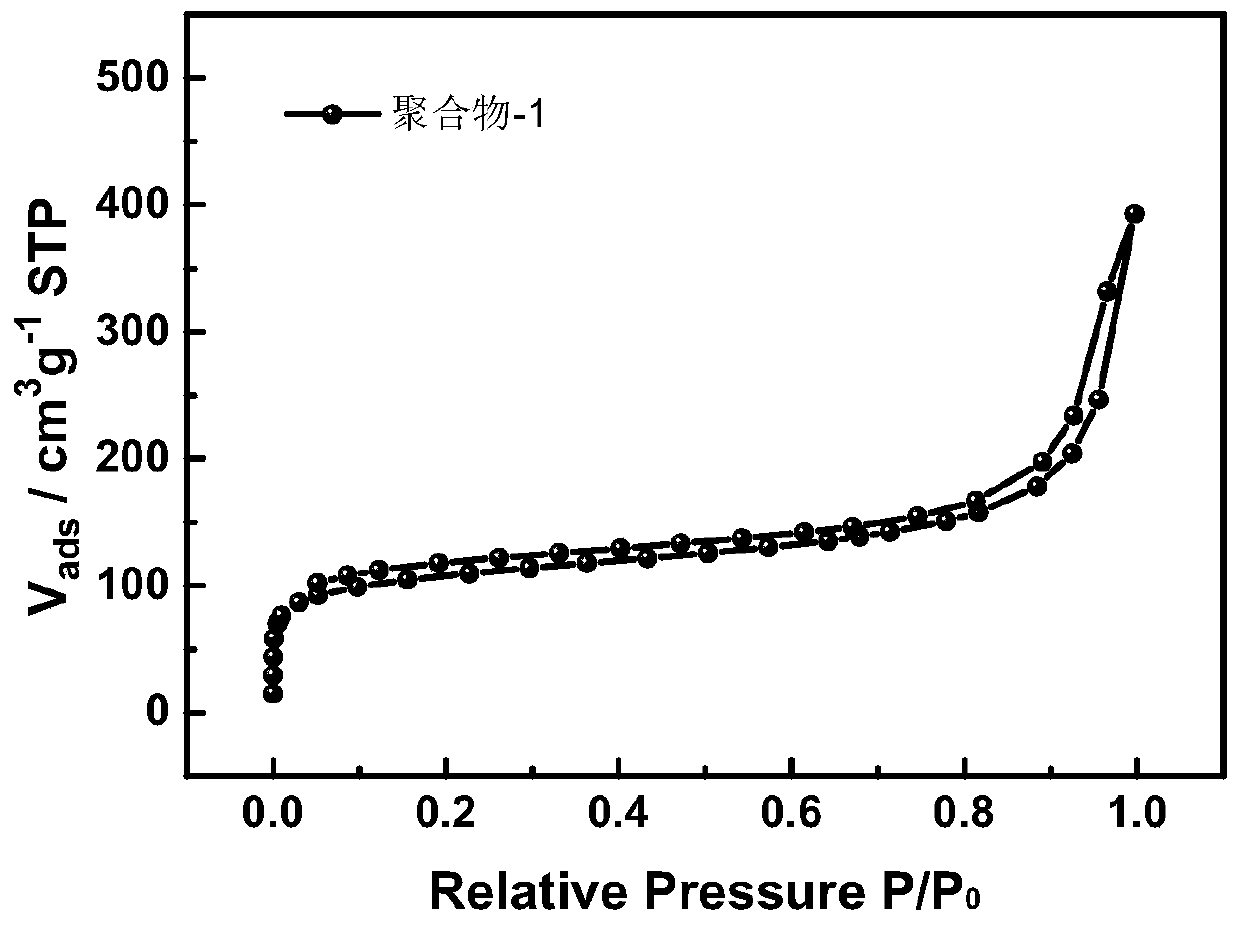
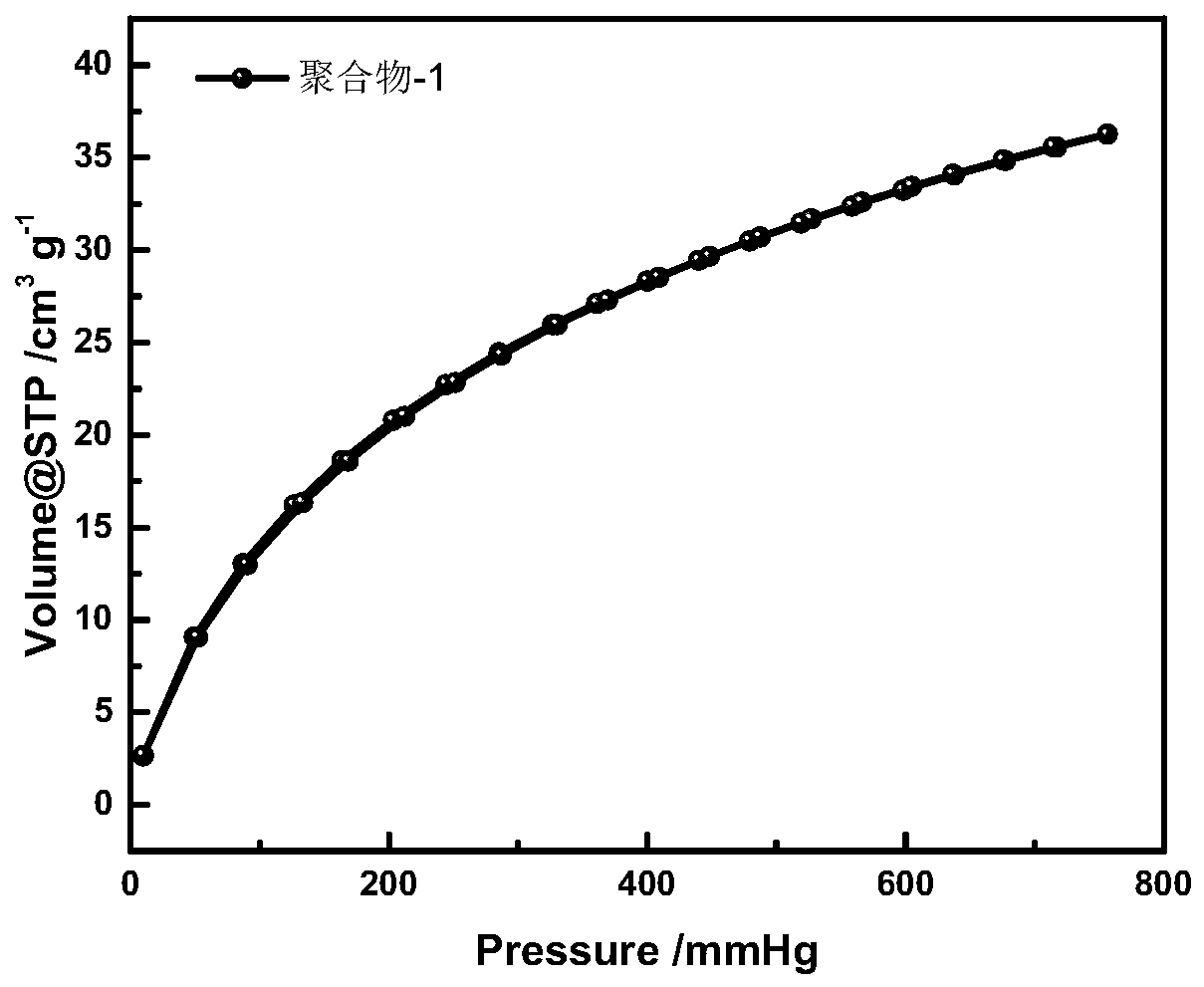
[0040] Mix 2,2'-bipyridine-5,5'-dicarbaldehyde (212.2mg, 1mmol) and 1,4-diacetylbenzene (324.4mg, 2mmol) in a 100ml round bottom flask, add ammonium acetate ( 2.31g, 30mmol), then 50ml of pyridine was added, and the mixture was placed in an oil bath at 115°C and stirred for 12h. After the reaction, filter with suction, wash with deionized water and chloroform at 60°C for 24 hours, and then dry in a vacuum oven at 100°C for 24 hours to obtain the pyridine / bipyridine conjugated microporous polymer, which is denoted as polymer Matter-1, its BET specific surface area is 347m 2 / g.
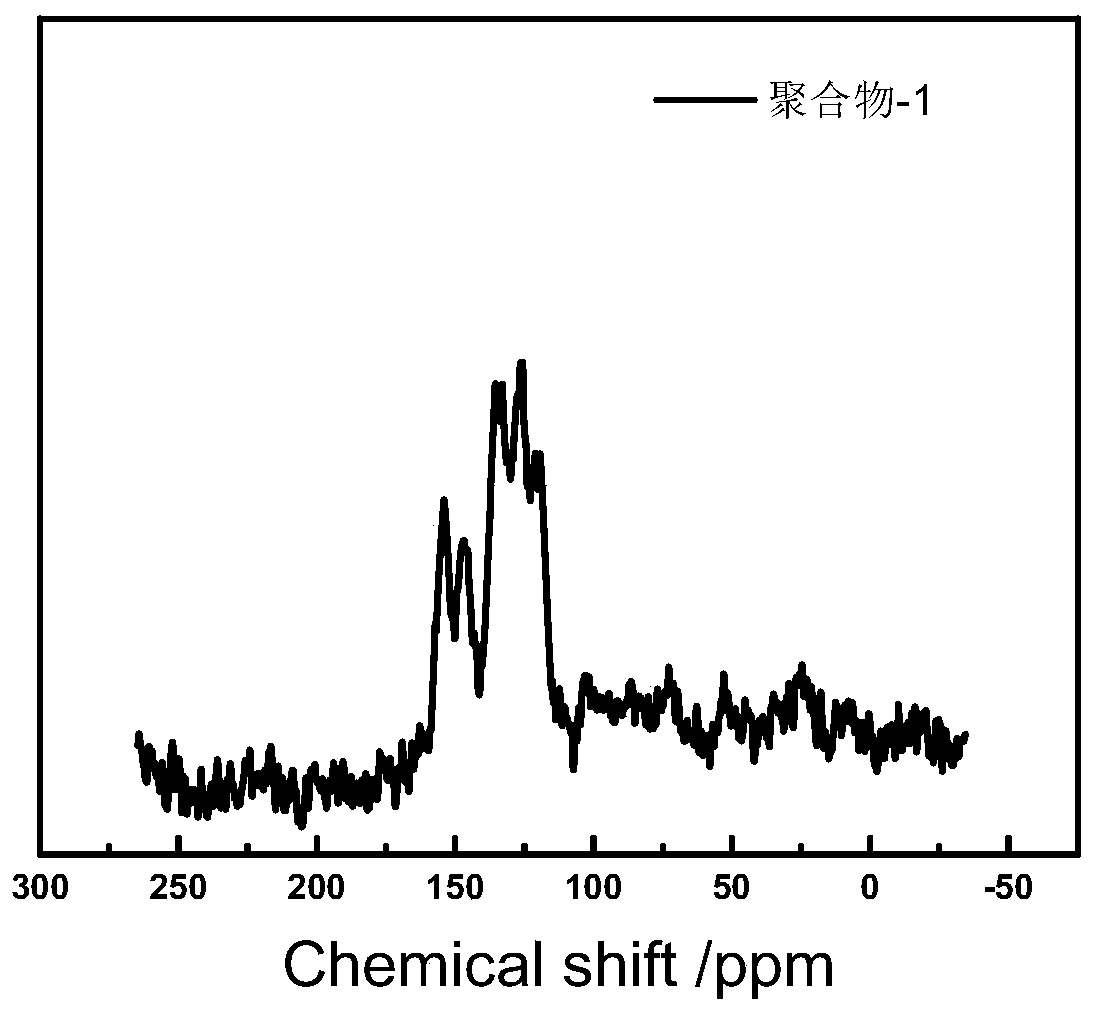
[0041] The polymer-1 that present embodiment obtains 13 C-NMR test results such as figure 1 As shown, it can be seen that the peaks at 153.8 and 146.1ppm chemical shifts are respectively the peaks of ortho and para C in the pyridyl chemical environment, proving the existence of pyridine nitrogen; the peaks at 134.6, 126.1 and 120.3ppm chemical shifts It is the peak of C in the chemical environment...
Embodiment 2
[0048] Mix 2,2'-bipyridine-5,5'-dicarbaldehyde (212.2mg, 1mmol) and 1,3-diacetylbenzene (324.4mg, 2mmol) in a 100ml round bottom flask, add ammonium acetate ( 2.31g, 30mmol), then add 25ml of acetic acid to dissolve the above mixed powder, and finally place the mixed solution in an oil bath at 115°C and stir for 12h. After the reaction, filter with suction, wash with deionized water and chloroform at 60°C for 24 hours, and then dry in a vacuum oven at 100°C for 24 hours to obtain the pyridine / bipyridine conjugated microporous polymer, which is denoted as polymer Matter-2, its BET specific surface area is 418m 2 / g.
[0049] The polymer-2 that present embodiment obtains 13 C-NMR test results such as Figure 7 As shown, it can be seen that the peaks at 155.6 and 148.5ppm chemical shifts are respectively the peaks of ortho and para C in the pyridyl chemical environment, proving the existence of pyridine nitrogen; the peaks at 135.6, 126.5 and 119.3ppm chemical shifts It is th...
Embodiment 3
[0056] Mix 2,2'-bipyridine-5,5'-dicarbaldehyde (212.2mg, 1mmol) and 2,6-diacetylpyridine (326.3mg, 2mmol) in a 100ml round bottom flask, add ammonium acetate ( 2.31g, 30mmol), then 50ml of pyridine was added, and finally the mixture was placed in an oil bath at 120°C and stirred for 12h. After the reaction, filter with suction, wash with deionized water and chloroform respectively at 60°C for 24 hours, and dry in a vacuum oven at 60°C for 24 hours after suction filtration to obtain the pyridine / bipyridine conjugated microporous polymer, which is denoted as polymer Matter-3, its BET specific surface area is 400m 2 / g.
[0057] The polymer-3 that present embodiment obtains 13 C-NMR test results such as Figure 13 As shown, it can be seen that the peaks at 154.5 and 148.2ppm chemical shifts are respectively the peaks of ortho and para C in the pyridyl chemical environment, proving the existence of pyridine nitrogen; the peaks at 135.3 and 120.0ppm chemical shifts are at The p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com