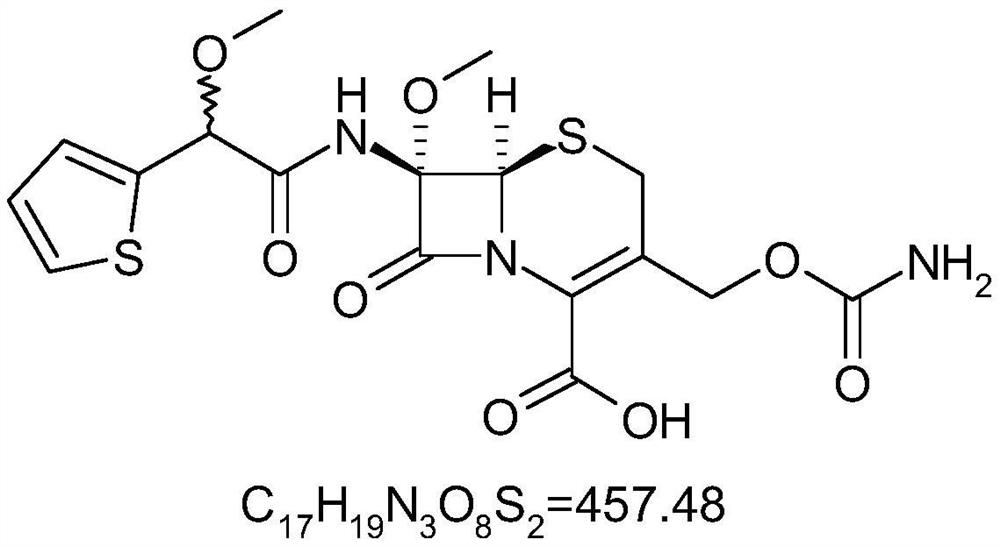
The preparation method of (rs)-methoxycefoxitin
A technology of methoxycefoxitin and methoxy, which is applied in the field of impurity analysis in drug synthesis, can solve problems such as toxic and side effects and affect drug efficacy, and achieve the effects of simple steps, guaranteed clinical safety and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
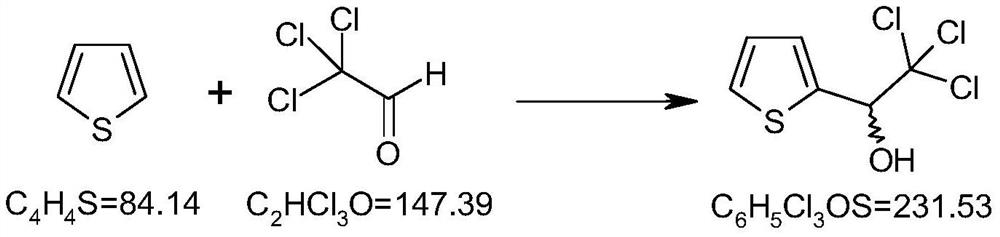
[0036] Chemical reaction formula for the preparation of (RS)-α-trichloromethyl-2-thiophene methanol
[0037]
[0038] In a 1000ml there-necked flask, add 84g (1.0mol) thiophene, 500ml n-heptane and 147g (1.0mol) trichloroacetaldehyde and stir, heat up to reflux, cool down to room temperature after the reaction, concentrate under reduced pressure to recover the solvent, and the residue is decompressed Distillation, collect 98~100 ℃, 1.0mmHg fraction, yield about 46g.
Embodiment 2
[0040] Preparation of (RS)-α-trichloromethyl-2-thiophene methanol
[0041] In a 1000ml there-necked flask, add 84g (1.0mol) thiophene, 500ml n-heptane and 147g (1.2mol) trichloroacetaldehyde and stir, heat up to reflux, cool down to room temperature after the reaction, concentrate under reduced pressure to recover the solvent, and the residue is decompressed Distillation, collect 98~100 ℃, 1.0mmHg fraction, yield about 52g.
Embodiment 3
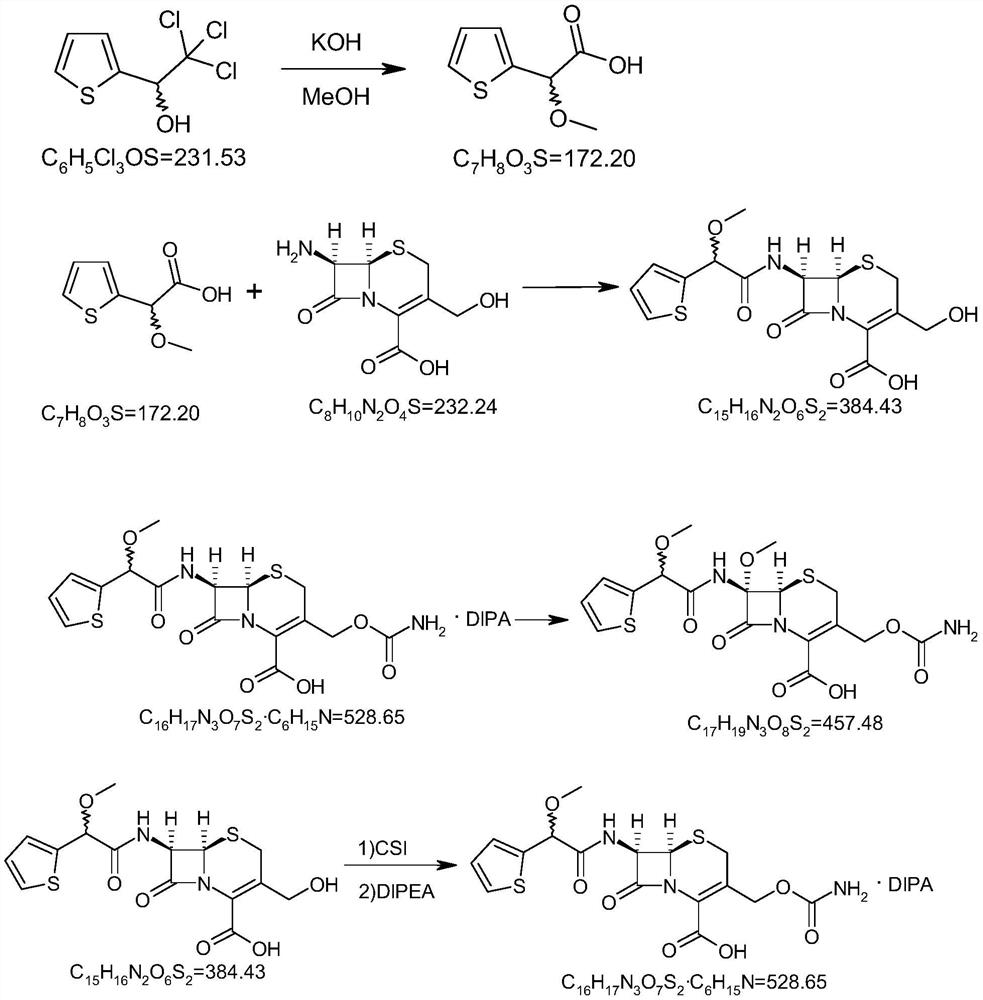
[0043] The chemical reaction formula for the preparation of (RS)-α-methoxy-2-thiophenephenylacetic acid
[0044]
[0045]Under nitrogen protection, add 100ml methanol and 11.2g (0.2mol) potassium hydroxide to a 500ml three-necked flask, stir to dissolve, cool to -5~0°C, add 11.6g (0.05mol) (R,S)-α-trimonium A solution of chloromethyl-2-thiophene methanol in 30 ml of methanol was stirred, slowly heated to reflux, cooled to room temperature after the reaction, concentrated under reduced pressure to remove the solvent, added 50 ml of methyl tert-butyl ether to the residue, stirred with 0.1N dilute hydrochloric acid was adjusted to pH 3.0, the organic layer was separated, the aqueous layer was extracted with methyl tert-butyl ether again, the organic layers were combined, washed with brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain about 6.2 g of product .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com