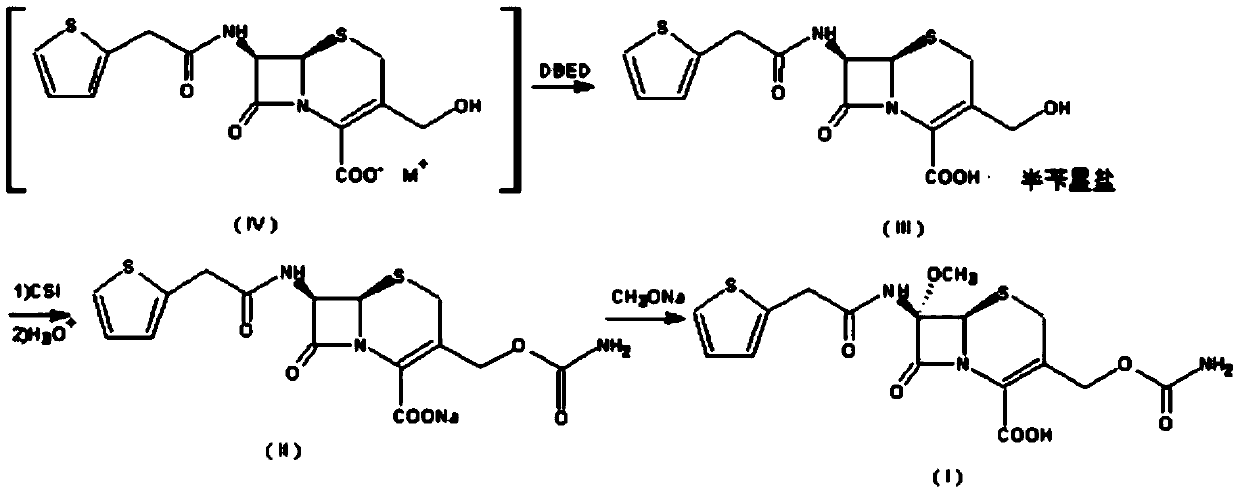
Preparation method of sulfamic acid methyl ester
The technology of methyl sulfamate and sulfamate chloride is applied in the field of impurity analysis in drug synthesis, which can solve problems such as affecting drug efficacy, toxic and side effects, and achieve the effects of clinical safety use guarantee, simple steps and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation method of methyl sulfamate
[0029] 1, the preparation of aminosulfonyl chloride
[0030]
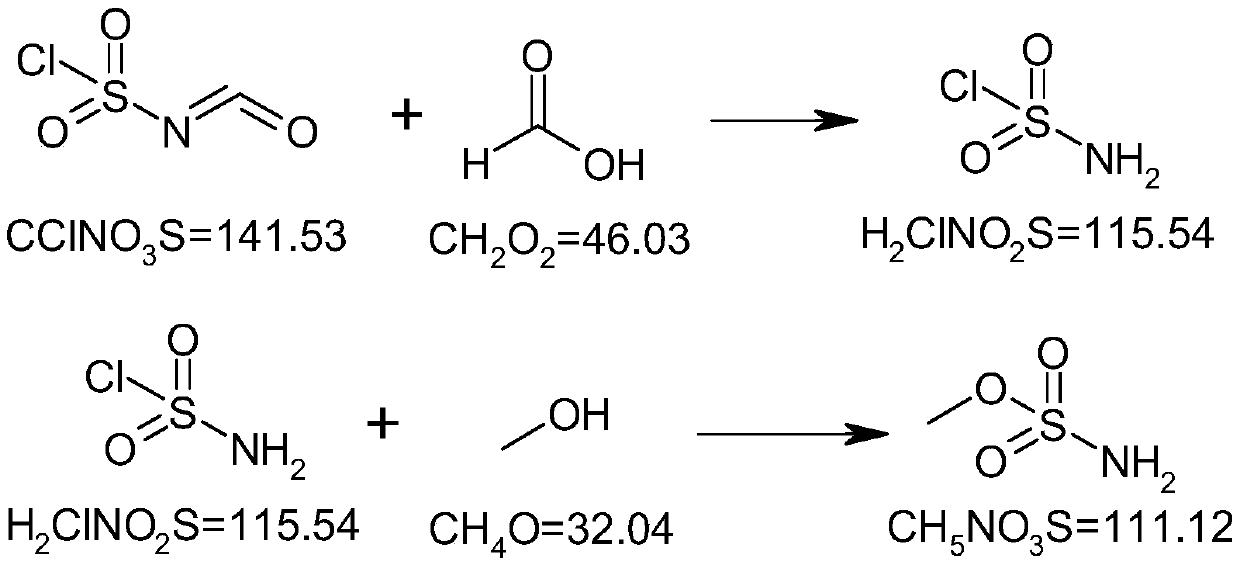
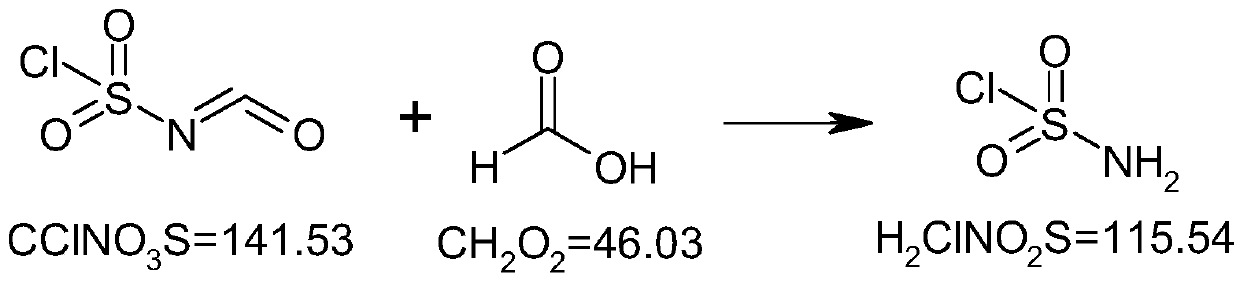
[0031] Add 141.5g (1.0mol) of chlorosulfonic acid isocyanate into a 500ml three-necked flask, protect it under nitrogen, stir, cool down to -15~-10°C, and add 49g (1.05mol) of formic acid dropwise at a temperature controlled below -10°C. The temperature was raised to room temperature and the stirring reaction was continued. After the reaction, add 200ml of dichloromethane, stir to dissolve, concentrate under reduced pressure and distill off the solvent, add 100ml of dichloromethane again, continue to concentrate under reduced pressure and distill off the residual solvent. The residue was suspended in petroleum ether (boiling point 60-90°C), stirred at room temperature under nitrogen protection, filtered under nitrogen protection, washed with petroleum ether, and the wet product was dried under reduced pressure below 35°C to obtain 107g of product with a yield ...
Embodiment 2
[0036] The preparation method of methyl sulfamate
[0037] 1, the preparation of aminosulfonyl chloride
[0038]
[0039] Add 141.5g (1.0mol) of chlorosulfonic acid isocyanate into a 500ml three-necked flask, protect with nitrogen, stir, cool down to -15~-10°C, control the temperature below -10°C and add 37.6g (0.8mol) formic acid dropwise. , warming up to room temperature and continuing to stir the reaction. After the reaction, add 200ml of chloroform, stir to dissolve, concentrate under reduced pressure and evaporate the solvent, add 100ml of chloroform again, continue to concentrate under reduced pressure and evaporate the residual solvent. The residue was suspended in methyl tert-butyl ether, stirred at room temperature under the protection of nitrogen, filtered under the protection of nitrogen, washed with methyl tert-butyl ether, and the wet product was dried under reduced pressure below 35°C to obtain 96.3g of the product, with a yield of 83.7% .
[0040] 2, the p...
Embodiment 3
[0044] The preparation method of methyl sulfamate
[0045] 1, the preparation of aminosulfonyl chloride
[0046]
[0047]Add 141.5g (1.0mol) of chlorosulfonic acid isocyanate into a 500ml three-necked flask, protect it under nitrogen, stir mechanically, cool down to -15~-10°C, and add 47g (1.0mol) of formic acid dropwise at a temperature below -10°C. , warming up to room temperature and continuing to stir the reaction. After the reaction, add 200ml of dichloroethane, stir to dissolve, concentrate under reduced pressure and evaporate the solvent, add 100ml of dichloroethane again, continue to concentrate under reduced pressure and evaporate the residual solvent. The residue was suspended in isopropyl ether, stirred at room temperature under the protection of nitrogen, filtered under the protection of nitrogen, washed with isopropyl ether, and the wet product was dried under reduced pressure below 35°C to obtain 109 g of the product, with a yield of 94.7%.
[0048] 2, the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com