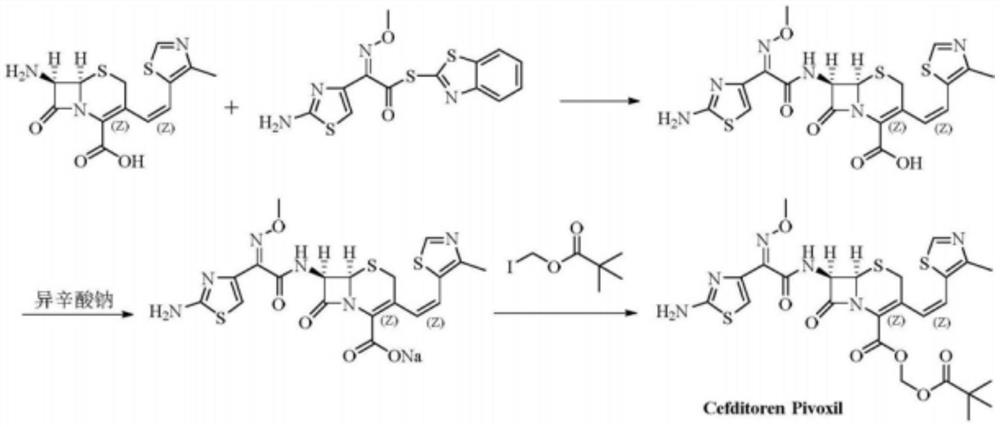
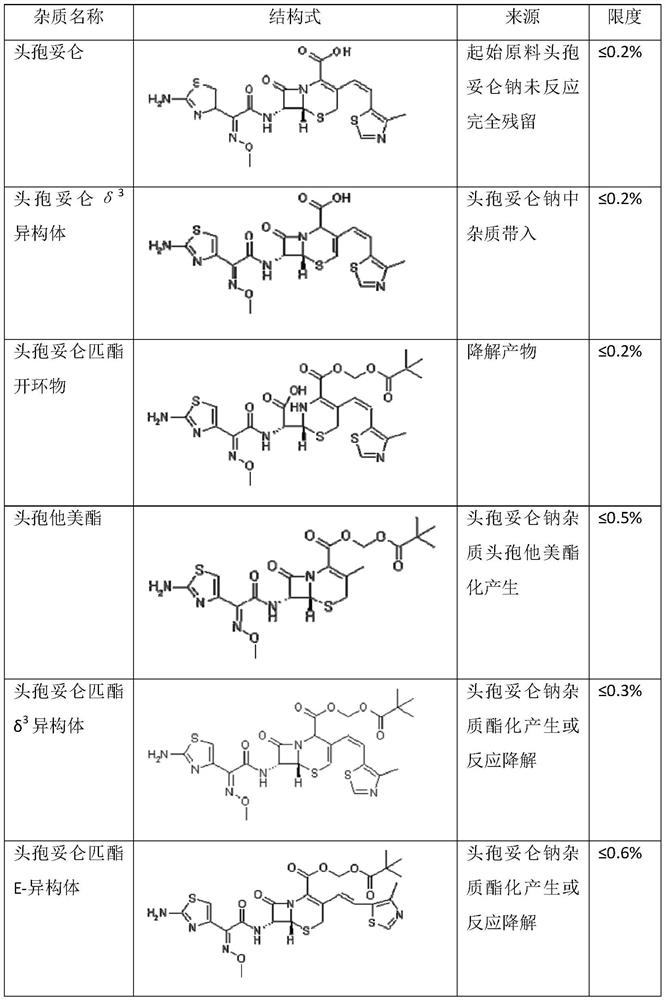
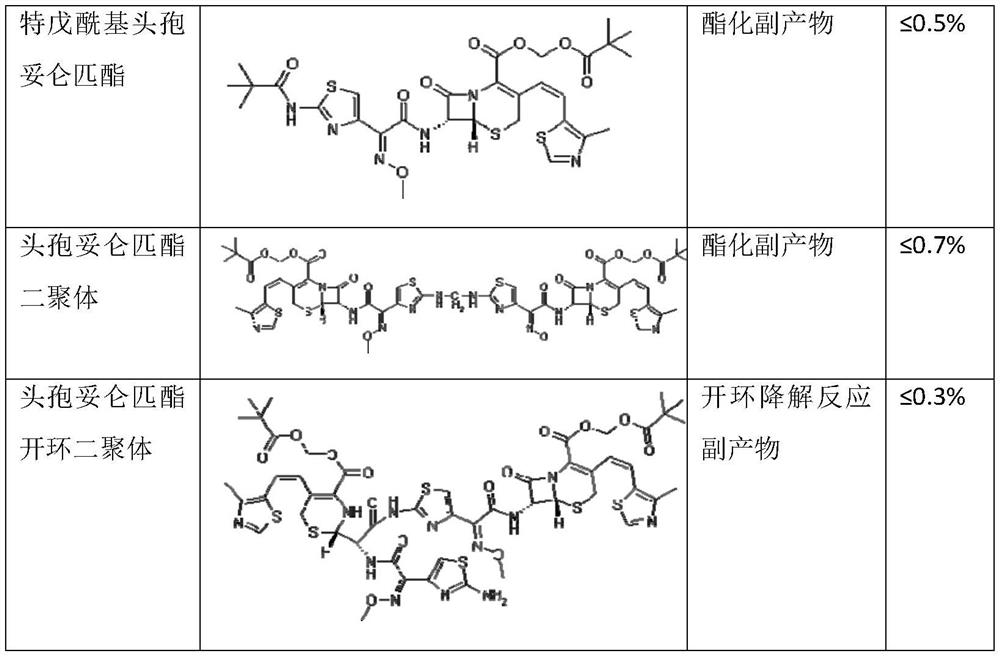
The preparation method of cefditoren pivoxil ring-opened product
A technology of cefditoren pivoxil and basic substances, which is applied in the field of impurity analysis in drug synthesis, can solve problems affecting accuracy, cumbersome steps, and difficult to completely separate impurities, and achieve low cost, simple steps, and clinical safety The effect of guarantee
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 6.2g of cefditoren pivoxil and 50ml of dimethyl sulfoxide into the reaction bottle, stir and cool down to -20°C, slowly add 0.01mol of sodium hydroxide, heat up to 10°C-20°C to react, after HPLC checks that the reaction is complete, Add 50ml of water and 50ml of ethyl acetate, adjust the pH value to 3-4 with 5% hydrochloric acid under stirring, let stand to separate the layers, extract the water layer with 20ml of ethyl acetate, combine the ethyl acetate layers, wash with saturated brine, anhydrous sulfuric acid Dried over magnesium, filtered, concentrated to dryness under reduced pressure, purified by silica gel column chromatography, eluted with ethyl acetate-petroleum ether (2:1), concentrated to obtain 4.5 g of light yellow solid. The purity of the product detected by HPLC was 97.8%, RRT0.56, which was consistent with the relative retention time of the ring-opened cefditoren pivoxil reference substance.
[0025] The product was analyzed by MS mass spectrometry ...
Embodiment 2
[0036] Add 6.2g of cefditoren pivoxil and 50ml of N,N-dimethylformamide into the reaction bottle, stir and cool down to -15°C, slowly add 0.012mol of potassium hydroxide, heat up to 10°C-20°C for reaction, and check by HPLC After the reaction is complete, add 50ml of water and 50ml of ethyl acetate, adjust the pH value to 3-4 with 10% hydrochloric acid under stirring, let the layers stand, extract the water layer with 20ml of ethyl acetate, combine the ethyl acetate layers, and wash with saturated brine , dried over anhydrous magnesium sulfate, filtered, concentrated to dryness under reduced pressure, separated by silica gel column chromatography, eluted with ethyl acetate-petroleum ether (2:1), and concentrated to obtain 4.5 g of a light yellow solid. The purity of the product detected by HPLC was 95.2%, and the RRT was 0.56, which was consistent with the relative retention time of the ring-opened cefditoren pivoxil reference substance. The product has passed MS, 1 H-NMR and...
Embodiment 3
[0038] Add 6.2g cefditoren pivoxil and 55ml N,N-dimethylacetamide into the reaction bottle, stir and cool down to -5°C, slowly add lithium hydroxide 0.011mol, heat up to 10°C-20°C to react, HPLC After checking that the reaction is complete, add 50ml of water and 50ml of ethyl acetate, adjust the pH value to 3-4 with 1% hydrochloric acid under stirring, let the layers stand, extract the water layer with 20ml of ethyl acetate, combine the ethyl acetate layers, and wash with saturated brine Washed, dried over anhydrous magnesium sulfate, filtered, concentrated to dryness under reduced pressure, purified by silica gel column chromatography, eluted with ethyl acetate-petroleum ether (2:1), concentrated to obtain 4.0 g of light yellow solid. The purity of the product detected by HPLC was 96.2%, and the RRT was 0.56, which was consistent with the relative retention time of the ring-opened cefditoren pivoxil reference substance. The product has passed MS, 1 H-NMR and 13 C NMR struct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com