(3R,4S)-1-carbobenzoxy-4-ethylpyrrole-3-carboxylic acid synthesis method suitable for industrialization
A technique for the synthesis of ethylpyrrole, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve problems such as difficult control and complicated production process, and achieve the effect of reducing preparation cost, reducing process steps, and easy control of operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
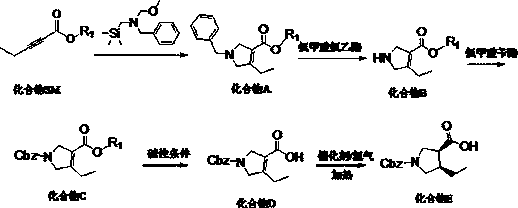
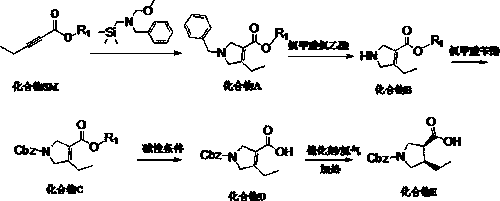
[0045] A synthetic method of (3R, 4S)-1-benzyloxycarbonyl-4-ethylpyrrole-3-carboxylic acid suitable for industrialization, characterized in that it includes the following steps:
[0046] S1, starting from 2-pentynoate and N-methoxymethyl-N-(trimethylsilylmethyl)benzylamine, the intermediate is prepared by condensation and ring-closure reaction under acid catalysis in a solvent Condensation intermediate product compound A, the structural formula of compound A is:
[0047] ;
[0048] S2, add compound A to the solvent, stir, add chloroethyl chloroformate at 0~5℃, react at 20~25℃ for 1~2h, add alcohol solvent, continue the reaction at 40~90℃, The intermediate product compound B is obtained, and the structural formula of the compound B is:
[0049] ;
[0050] S3: Under alkaline conditions, compound B is prepared by acylation reaction with a protecting group reagent in a solvent to obtain N-position protected intermediate product compound C. The structural formula of compound C is:
[0051...
Embodiment 1
[0060] S1, add 50.0g ethyl pentynoate (1.0eq, 0.396mol) into the reaction flask, add 141.15g (1.5eq, 0.594mol) N-(methoxymethyl)-N-(trimethylsilylmethyl) ) Benzylamine, add 750 mL of acetonitrile to the above reaction flask, add concentrated hydrochloric acid / aqueous solution 3.8g / 20ml (0.1eq, 0.0396mol) dropwise at room temperature, and the addition is completed in about 30min. Keep at room temperature 20~25℃ to react for 16h.
[0061] Post-treatment: After the reaction, concentrate to remove acetonitrile, add 700ml ethyl acetate, wash twice with saturated aqueous sodium bicarbonate solution (250mL×2), and then wash once with aqueous sodium chloride solution (250mL×1); reduce the organic phase Distill until no fraction is produced (40~50℃, -0.1Mpa), and use it directly in the next step.
[0062] The reaction equation is:
[0063] .
[0064] S2, put the crude compound A (1.0eq, 0.396mol) and 1500mL of dichloromethane into the reaction flask and stir until the solution is clear; add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com